Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury
- PMID: 14970018
- PMCID: PMC7974622
Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury
Abstract
Background and purpose: Diffusion-weighted (DW) MR imaging is a means to characterize and differentiate morphologic features, including edema, necrosis, and tumor tissue, by measuring differences in apparent diffusion coefficient (ADC). We hypothesized that DW imaging has the potential to differentiate recurrent or progressive tumor growth from treatment-induced damage to brain parenchyma in high-grade gliomas after radiation therapy.
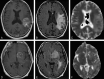
Methods: We retrospectively reviewed follow-up conventional and DW MR images obtained starting 1 month after completion of radiation treatment with or without chemotherapy for histologically proved high-grade gliomas. Eighteen patients with areas of abnormal enhancing tissue were identified. ADC maps were calculated from echo-planar DW images, and mean ADC values and ADC ratios (ADC of enhancing lesion to ADC of contralateral white matter) were compared with final diagnosis. Recurrence was established by histologic examination or by clinical course and a combination of imaging studies.
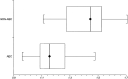
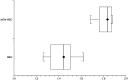
Results: Recurrence and nonrecurrence could be differentiated by using mean ADC values and ADC ratios. ADC ratios in the recurrence group showed significantly lower values (mean +/- SD, 1.43 +/- 0.11) than those of the nonrecurrence group (1.82 +/- 0.07, P <.001). Mean ADCs of the recurrent tumors (mean +/- SD, 1.18 +/- 0.13 x 10(-3) mm/s(2)) were significantly lower than those of the nonrecurrence group (1.40 +/- 0.17 x 10(-3) mm/s(2), P <.006).
Conclusion: Assessment of ADC ratios of enhancing regions in the follow-up of treated high-grade gliomas is useful in differentiating radiation effects from tumor recurrence or progression.
Figures



References
-
- Samnick S, Bader JB, Hellwig D, et al. Clinical value of iodine-123-alpha-methyl-L-tyrosine single-photon emission tomography in the differential diagnosis of recurrent brain tumor in patients pretreated for glioma at follow-up. J Clin Oncol 2002;20:396–404 - PubMed
-
- Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 2000;41:1861–1867 - PubMed
-
- Schlemmer HP, Bachert P, Henze M, et al. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology 2002;44:216–222 - PubMed
-
- Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377–384 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
