Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1
- PMID: 14970176
- PMCID: PMC2211834
- DOI: 10.1084/jem.20030437
Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1
Abstract
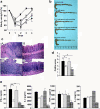
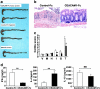
Carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1) is a cell surface molecule that has been proposed to negatively regulate T cell function. We have shown that CEACAM1 is associated with specific regulation of T helper cell (Th)1 pathways, T-bet-mediated Th1 cytokine signaling, and Th1-mediated immunopathology in vivo. Mice treated with anti-mouse CEACAM1-specific monoclonal antibody (mAb) CC1 during the effector phase exhibited a reduced severity of trinitrobenzene sulfonic acid colitis in association with decreased interferon (IFN)-gamma production. Although oxazolone colitis has been reported as Th2 mediated, mice treated with the CC1 mAb or a CEACAM1-Fc chimeric protein exhibited a reduced severity of colitis in association with a significant reduction of IFN-gamma and T-bet activation, whereas signal transducer and activator of antigen 4 activation was unaffected. Both interleukin-4 and IFN-gamma gene-deficient mice exhibited less severe colitis induction by oxazolone. Direct ligation of T cells in vitro with the murine hepatitis virus spike protein, a natural ligand for the N-domain of CEACAM1, inhibited the differentiation of naive cells into Th1 but not Th2 cells and activation of Th1 but not Th2 cytokine production. These results indicate that CEACAM1 isoforms are a novel class of activation-induced cell surface molecules on T cells that function in the specific regulation of Th1-mediated inflammation such as that associated with inflammatory bowel disease.
Figures



References
-
- Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K.V. Holmes, A. Karlsson, M. Kuroki, et al. 1999. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243–249. - PubMed
-
- Hammarstrom, S. 1999. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9:67–81. - PubMed
-
- Kammerer, R., D. Stober, B.B. Singer, B. Obrink, and J. Reimann. 2001. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J. Immunol. 166:6537–6544. - PubMed
-
- Moller, M.J., R. Kammerer, F. Grunert, and S. von Kleist. 1996. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int. J. Cancer. 65:740–745. - PubMed
-
- Chen, T., W. Zimmermann, J. Parker, I. Chen, A. Maeda, and S. Bolland. 2001. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J. Leukoc. Biol. 70:335–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

