Chronological aging leads to apoptosis in yeast
- PMID: 14970189
- PMCID: PMC2171996
- DOI: 10.1083/jcb.200310014
Chronological aging leads to apoptosis in yeast
Abstract
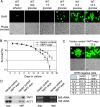
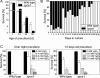
During the past years, yeast has been successfully established as a model to study mechanisms of apoptotic regulation. However, the beneficial effects of such a cell suicide program for a unicellular organism remained obscure. Here, we demonstrate that chronologically aged yeast cultures die exhibiting typical markers of apoptosis, accumulate oxygen radicals, and show caspase activation. Age-induced cell death is strongly delayed by overexpressing YAP1, a key transcriptional regulator in oxygen stress response. Disruption of apoptosis through deletion of yeast caspase YCA1 initially results in better survival of aged cultures. However, surviving cells lose the ability of regrowth, indicating that predamaged cells accumulate in the absence of apoptotic cell removal. Moreover, wild-type cells outlast yca1 disruptants in direct competition assays during long-term aging. We suggest that apoptosis in yeast confers a selective advantage for this unicellular organism, and demonstrate that old yeast cells release substances into the medium that stimulate survival of the clone.
Copyright The Rockefeller University Press
Figures


References
-
- Jamieson, D.J., S.L. Rivers, and D.W. Stephen. 1994. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology. 140:3277–3283. - PubMed
-
- Jungwirth, H., H. Bergler, and G. Högenauer. 2001. Diazaborine treatment of Baker's yeast results in stabilization of aberrant mRNAs. J. Biol. Chem. 276:36419–36424. - PubMed
-
- Laun, P., A. Pichova, F. Madeo, J. Fuchs, A. Ellinger, S. Kohlwein, I. Dawes, K.U. Fröhlich, and M. Breitenbach. 2001. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 39:1166–1173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

