Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia
- PMID: 14970327
- PMCID: PMC373506
- DOI: 10.1073/pnas.0308750101
Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia
Abstract
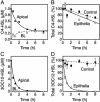
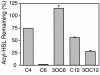
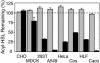
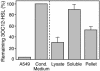
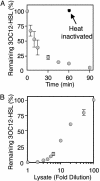
Mammalian airways protect themselves from bacterial infection by using multiple defense mechanisms including antimicrobial peptides, mucociliary clearance, and phagocytic cells. We asked whether airways might also target a key bacterial cell-cell communication system, quorum-sensing. The opportunistic pathogen Pseudomonas aeruginosa uses two quorum-sensing molecules, N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), to control production of extracellular virulence factors and biofilm formation. We found that differentiated human airway epithelia inactivated 3OC12-HSL. Inactivation was selective for acyl-HSLs with certain acyl side chains, and C4-HSL was not inactivated. In addition, the capacity for inactivation varied widely in different cell types. 3OC12-HSL was inactivated by a cell-associated activity rather than a secreted factor. These data suggest that the ability of human airway epithelia to inactivate quorum-sensing signal molecules could play a role in the innate defense against bacterial infection.
Figures
Comment in
-
Bacterial quorum-sensing signals are inactivated by mammalian cells.Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):3993-4. doi: 10.1073/pnas.0400874101. Epub 2004 Mar 15. Proc Natl Acad Sci U S A. 2004. PMID: 15024108 Free PMC article. No abstract available.
Similar articles
-
Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia.Am J Physiol Lung Cell Mol Physiol. 2007 Apr;292(4):L852-60. doi: 10.1152/ajplung.00370.2006. Epub 2006 Nov 22. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17122353
-
Correlation Between Quorum Sensing Signal Molecules and Pseudomonas aeruginosa's Biofilm Development and Virulency.Curr Microbiol. 2018 Jul;75(7):787-793. doi: 10.1007/s00284-018-1449-5. Epub 2018 Feb 9. Curr Microbiol. 2018. PMID: 29427006
-
The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo.J Bacteriol. 2002 Feb;184(4):1132-9. doi: 10.1128/jb.184.4.1132-1139.2002. J Bacteriol. 2002. PMID: 11807074 Free PMC article.
-
Bacteria-Host Crosstalk: Sensing of the Quorum in the Context of Pseudomonas aeruginosa Infections.J Innate Immun. 2019;11(3):263-279. doi: 10.1159/000494069. Epub 2018 Nov 14. J Innate Immun. 2019. PMID: 30428481 Free PMC article. Review.
-
Quorum sensing: the power of cooperation in the world of Pseudomonas.Environ Microbiol. 2005 Apr;7(4):459-71. doi: 10.1111/j.1462-2920.2005.00769.x. Environ Microbiol. 2005. PMID: 15816912 Review.
Cited by
-
Expression of human paraoxonase 1 decreases superoxide levels and alters bacterial colonization in the gut of Drosophila melanogaster.PLoS One. 2012;7(8):e43777. doi: 10.1371/journal.pone.0043777. Epub 2012 Aug 30. PLoS One. 2012. PMID: 22952763 Free PMC article.
-
Molecular Mechanisms Involved in Pseudomonas aeruginosa Bacteremia.Adv Exp Med Biol. 2022;1386:325-345. doi: 10.1007/978-3-031-08491-1_12. Adv Exp Med Biol. 2022. PMID: 36258078 Review.
-
Quorum quenching: Signal jamming in dental plaque biofilms.J Dent Sci. 2016 Dec;11(4):349-352. doi: 10.1016/j.jds.2016.02.002. Epub 2016 Apr 11. J Dent Sci. 2016. PMID: 30894996 Free PMC article. Review.
-
Friends or Foes-Microbial Interactions in Nature.Biology (Basel). 2021 Jun 2;10(6):496. doi: 10.3390/biology10060496. Biology (Basel). 2021. PMID: 34199553 Free PMC article. Review.
-
Effect of Sub-Minimum Inhibitory Concentrations of Tyrosol and EDTA on Quorum Sensing and Virulence of Pseudomonas aeruginosa.Infect Drug Resist. 2020 Oct 8;13:3501-3511. doi: 10.2147/IDR.S264805. eCollection 2020. Infect Drug Resist. 2020. PMID: 33116669 Free PMC article.
References
-
- Fuqua, C. & Greenberg, E. P. (2002) Nat. Rev. Mol. Cell Biol. 3, 685-695. - PubMed
-
- Fuqua, C., Parsek, M. R. & Greenberg, E. P. (2001) Annu. Rev. Genet. 35, 439-468. - PubMed
-
- Whitehead, N. A., Barnard, A. M., Slater, H., Simpson, N. J. & Salmond, G. P. (2001) FEMS Microbiol. Rev. 25, 365-404. - PubMed
-
- Whitehead, N. A., Byers, J. T., Commander, P., Corbett, M. J., Coulthurst, S. J., Everson, L., Harris, A. K., Pemberton, C. L., Simpson, N. J., Slater, H., et al. (2002) Antonie Leeuwenhoek 81, 223-231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

