Characterization of deadenylation in trypanosome extracts and its inhibition by poly(A)-binding protein Pab1p
- PMID: 14970390
- PMCID: PMC1370940
- DOI: 10.1261/rna.5180304
Characterization of deadenylation in trypanosome extracts and its inhibition by poly(A)-binding protein Pab1p
Abstract
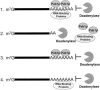
The stability of mRNAs is an important point in the regulation of gene expression in eukaryotes. The mRNA turnover pathways have been identified in yeast and mammals. However, mRNA turnover pathways in trypanosomes have not been widely studied. Deadenylation is the first step in the major mRNA turnover pathways of yeast and mammals. To better understand mRNA degradation processes in these organisms, we have developed an in vitro mRNA turnover system that is functional for deadenylation. In this system, addition of poly(A) homopolymer activates the deadenylation of poly(A) tails. The trypanosomal deadenylase activity is a 3'-->5' exonuclease specific for adenylate residues, generates 5'-AMP as a product, is magnesium dependent, and is inhibited by neomycin B sulfate. These characteristics suggest similarity with other eukaryotic deadenylases. Furthermore, this activity is cap independent, indicating a potential difference between the trypanosomal activity and PARN, but suggesting similarity to Ccr4p/Pop2p activities. Extracts immunodepleted of Pab1p required the addition of poly(A) competition to activate deadenylation. Trypanosomal Pab1p functions as an inhibitor of the activity under in vitro conditions. Pab1p appears to be one of several mRNA stability proteins in trypanosomal extracts.
Figures





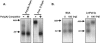
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
