Interleukin-2 improves tumour response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma
- PMID: 14970852
- PMCID: PMC2410164
- DOI: 10.1038/sj.bjc.6601563
Interleukin-2 improves tumour response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma
Abstract
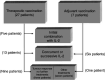
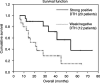
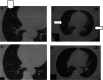
This paper is a report of response rate (RR) and survival of 34 metastatic melanoma patients who received a dinitrophenyl (DNP)-modified autologous melanoma cell vaccine. In all, 27 patients started the vaccine as a primary treatment for metastatic melanoma and seven started it as an adjuvant, with no evidence of disease at the time, but had developed new metastases. Interleukin-2 (IL-2) was administered in 24 out of the 34 patients: 19 who progressed on vaccine alone and five who had the combination from start. Interleukin-2 was administered in the intravenous, bolus high-dose regimen (seven patients) or as subcutaneous (s.c.) low-dose treatment (17). Overall response for the entire group was 35% (12 patients out of 34), 12% having a complete response (CR) and 23% a partial response (PR). However, only two patients had tumour responses while on the vaccine alone, whereas the other 10 demonstrated objective tumour regression following the combination with IL-2 (two CR, eight PR), lasting for a median duration of 6 months (range 3-50 months). Of the 12 responding patients, 11 attained strong skin reactivity to the s.c. injection of irradiated, unmodified autologous melanoma cells. None of the patients with a negative reactivity experienced any tumour response. Patients with positive skin reactions survived longer (median survival - 54 months). The results suggest enhanced RRs to the combination of IL-2 and autologous melanoma vaccine. Skin reactivity to unmodified autologous melanoma cells may be a predictor of response and improved survival, and therefore a criterion for further pursuing of immunotherapeutic strategies.
Figures


References
-
- Atkins MB, Kunkel L, Sznol M, Rosenberg SA (2000) High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 6(Suppl 1): S11–S14 - PubMed
-
- Atzpodien J, Neuber K, Kamanabrou D, Fluck M, Brocker EB, Neumann C, Runger TM, Schuler G, von den Driesch P, Muller I, Paul E, Patzelt T, Reitz M (2002) Combination chemotherapy with or without s.c. IL-2 and IFN-alpha: results of a prospectively randomized trial of the Cooperative Advanced Malignant Melanoma Chemoimmunotherapy Group (ACIMM). Br J Cancer 86: 179–184 - PMC - PubMed
-
- Baars A, Claessen AM, van den Eertwegh AJ, Gall HE, Stam AG, Meijer S, Giaccone G, Meijer CJ, Scheper RJ, Wagstaff J, Vermorken JB, Pinedo HM (2000) Skin tests predict survival after autologous tumor cell vaccination in metastatic melanoma: experience in 81 patients. Ann Oncol 11: 965–970 - PubMed
-
- Barth RJ, Mule JJ (2000) Interleukin-2: preclinical trials. In Principles and Practice of the Biologic Therapy of Cancer, Rosenberg SA (ed) pp 19–31. Philadelphia: Lippincott Williams & Wilkins
-
- Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, Gallino G, Piris A, Cattelan A, Lazzari I, Carrabba M, Scita G, Santantonio C, Pilla L, Tragni G, Lombardo C, Arienti F, Marchiano A, Queirolo P, Bertolini F, Cova A, Lamaj E, Ascani L, Camerini R, Corsi M, Cascinelli N, Lewis JJ, Srivastava P, Parmiani G (2002) Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96–peptide complexes: clinical and immunologic findings. J Clin Oncol 20: 4169–4180 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

