A dominant-negative fur mutation in Bradyrhizobium japonicum
- PMID: 14973020
- PMCID: PMC344408
- DOI: 10.1128/JB.186.5.1409-1414.2004
A dominant-negative fur mutation in Bradyrhizobium japonicum
Abstract
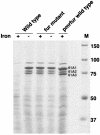
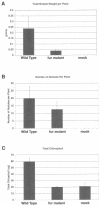
In many bacteria, the ferric uptake regulator (Fur) protein plays a central role in the regulation of iron uptake genes. Because iron figures prominently in the agriculturally important symbiosis between soybean and its nitrogen-fixing endosymbiont Bradyrhizobium japonicum, we wanted to assess the role of Fur in the interaction. We identified a fur mutant by selecting for manganese resistance. Manganese interacts with the Fur protein and represses iron uptake genes. In the presence of high levels of manganese, bacteria with a wild-type copy of the fur gene repress iron uptake systems and starve for iron, whereas fur mutants fail to repress iron uptake systems and survive. The B. japonicum fur mutant, as expected, fails to repress iron-regulated outer membrane proteins in the presence of iron. Unexpectedly, a wild-type copy of the fur gene cannot complement the fur mutant. Expression of the fur mutant allele in wild-type cells leads to a fur phenotype. Unlike a B. japonicum fur-null mutant, the strain carrying the dominant-negative fur mutation is unable to form functional, nitrogen-fixing nodules on soybean, mung bean, or cowpea, suggesting a role for a Fur-regulated protein or proteins in the symbiosis.
Figures

Similar articles
-
Identification of a functional fur gene in Bradyrhizobium japonicum.J Bacteriol. 1999 Sep;181(18):5843-6. doi: 10.1128/JB.181.18.5843-5846.1999. J Bacteriol. 1999. PMID: 10482529 Free PMC article.
-
Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum.Mol Microbiol. 2001 Aug;41(4):787-800. doi: 10.1046/j.1365-2958.2001.02555.x. Mol Microbiol. 2001. PMID: 11532144
-
An iron uptake operon required for proper nodule development in the Bradyrhizobium japonicum-soybean symbiosis.Mol Plant Microbe Interact. 2005 Sep;18(9):950-9. doi: 10.1094/MPMI-18-0950. Mol Plant Microbe Interact. 2005. PMID: 16167765
-
Living without Fur: the subtlety and complexity of iron-responsive gene regulation in the symbiotic bacterium Rhizobium and other alpha-proteobacteria.Biometals. 2007 Jun;20(3-4):501-11. doi: 10.1007/s10534-007-9085-8. Epub 2007 Feb 20. Biometals. 2007. PMID: 17310401 Review.
-
Members of the Fur protein family regulate iron and zinc transport in E. coli and characteristics of the Fur-regulated fhuF protein.J Mol Microbiol Biotechnol. 2002 May;4(3):217-22. J Mol Microbiol Biotechnol. 2002. PMID: 11931550 Review.
Cited by
-
The Bradyrhizobium japonicum Fur protein is an iron-responsive regulator in vivo.Mol Genet Genomics. 2006 Dec;276(6):555-64. doi: 10.1007/s00438-006-0162-4. Epub 2006 Oct 13. Mol Genet Genomics. 2006. PMID: 17039378
-
Metal homeostasis and resistance in bacteria.Nat Rev Microbiol. 2017 Jun;15(6):338-350. doi: 10.1038/nrmicro.2017.15. Epub 2017 Mar 27. Nat Rev Microbiol. 2017. PMID: 28344348 Free PMC article. Review.
-
Positive control of ferric siderophore receptor gene expression by the Irr protein in Bradyrhizobium japonicum.J Bacteriol. 2009 Mar;191(5):1361-8. doi: 10.1128/JB.01571-08. Epub 2008 Dec 29. J Bacteriol. 2009. PMID: 19114488 Free PMC article.
-
Iron-dependent cytochrome c1 expression is mediated by the status of heme in Bradyrhizobium japonicum.J Bacteriol. 2005 Aug;187(15):5084-9. doi: 10.1128/JB.187.15.5084-5089.2005. J Bacteriol. 2005. PMID: 16030200 Free PMC article.
-
Interplay between iron homeostasis and the osmotic stress response in the halophilic bacterium Chromohalobacter salexigens.Appl Environ Microbiol. 2010 Jun;76(11):3575-89. doi: 10.1128/AEM.03136-09. Epub 2010 Apr 2. Appl Environ Microbiol. 2010. PMID: 20363778 Free PMC article.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2003. Current protocols in molecular biology. Greene Publishing Associates and John Wiley & Sons, Inc., New York.
-
- Baichoo, N., T. Wang, R. Ye, and J. Helmann. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45:1613-1629. - PubMed
-
- De Luca, N. G., and A. W. B. Johnston. 1998. Is the fur gene of Rhizobium leguminosarum essential? FEMS Microbiol. Lett. 168:289-295. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

