Imaging tri-fusion multimodality reporter gene expression in living subjects
- PMID: 14973078
- PMCID: PMC4154814
- DOI: 10.1158/0008-5472.can-03-1816
Imaging tri-fusion multimodality reporter gene expression in living subjects
Abstract
Imaging reporter gene expression in living subjects with various imaging modalities is a rapidly accelerating area of research. Applications of these technologies to cancer research, gene therapy, and transgenic models are rapidly expanding. We report construction and testing of several triple fusion reporter genes compatible with bioluminescence, fluorescence and positron emission tomography (PET) imaging. A triple fusion reporter vector harboring a bioluminescence synthetic Renilla luciferase (hrl) reporter gene, a reporter gene encoding the monomeric red fluorescence protein (mrfp1), and a mutant herpes simplex virus type 1 sr39 thymidine kinase [HSV1-truncated sr39tk (ttk); a PET reporter gene] was found to preserve the most activity for each protein component and was therefore investigated in detail. After validating the activities of all three proteins encoded by the fusion gene in cell culture, we imaged living mice bearing 293T cells transiently expressing the hrl-mrfp-ttk vector by microPET and using a highly sensitive cooled charge-coupled device camera compatible with both bioluminescence and fluorescence imaging. A lentiviral vector carrying the triple fusion reporter gene was constructed and used to isolate stable expressers by fluorescence-activated cell sorting. These stable 293T cells were further used to show good correlation (R(2) approximately 0.74-0.85) of signal from each component by imaging tumor xenografts in living mice with all three modalities. Furthermore, metastases of a human melanoma cell line (A375M) stably expressing the triple fusion were imaged by microPET and optical technologies over a 40-50-day time period in living mice. Imaging of reporter gene expression from single cells to living animals with the help of a single tri-fusion reporter gene will have the potential to accelerate translational cancer research.
Figures
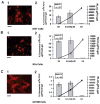
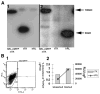

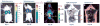
References
-
- Massoud T, Gambhir S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. - PubMed
-
- Van Roessel P, Brand A. Imaging into the future: visualizing gene expression and protein interactions with fluorescent proteins. Nat Cell Biol. 2002;4:E15–E20. - PubMed
-
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. - PubMed
-
- Bremer C, Weissleder R. In vivo imaging of gene expression. Acad Radiol. 2001;8:15–23. - PubMed
-
- Vooijs M, Jonkers J, Lyons S, Berns A. Noninvasive imaging of spontaneous retinoblastoma pathway-dependent tumors in mice. Cancer Res. 2002;62:1862–1867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

