Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum
- PMID: 14973161
- PMCID: PMC385276
- DOI: 10.1105/tpc.019398
Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum
Abstract
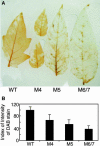
Plant respiratory burst oxidase homologs (Rboh) are homologs of the human neutrophil pathogen-related gp91(phox). Antisense technology was employed to ascertain the biological function of Lycopersicon esculentum (tomato) Rboh. Lines with diminished Rboh activity showed a reduced level of reactive oxygen species (ROS) in the leaf, implying a role for Rboh in establishing the cellular redox milieu. Surprisingly, the antisense plants acquired a highly branched phenotype, switched from indeterminate to determinate growth habit, and had fasciated reproductive organs. Wound-induced systemic expression of proteinase inhibitor II was compromised in the antisense lines, indicating that ROS intermediates supplied by Rboh are required for this wound response. Extending these observations by transcriptome analysis revealed ectopic leaf expression of homeotic MADS box genes that are normally expressed only in reproductive organs. In addition, both Rboh-dependent and -independent wound-induced gene induction was detected as well as transcript changes related to redox maintenance. The results provide novel insights into how the steady state cellular level of ROS is controlled and portrays the role of Rboh as a signal transducer of stress and developmental responses.
Figures







References
-
- Ahmad, K., and Henikoff, S. (2002). The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200. - PubMed
-
- Baker, C.J., and Orlandi, E.W. (1995). Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33, 299–321. - PubMed
-
- Banfi, B., Molnar, G., Maturana, A., Steger, K., Hegedus, B., Demaurex, N., and Krause, K.H. (2001). A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276, 37594–37601. - PubMed
-
- Bokoch, G.M., Bohl, B.P., and Chuang, T.H. (1994). Guanine-nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem. 269, 31674–31679. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

