Gene transfer in the evolution of parasite nucleotide biosynthesis
- PMID: 14973196
- PMCID: PMC365759
- DOI: 10.1073/pnas.0304686101
Gene transfer in the evolution of parasite nucleotide biosynthesis
Abstract
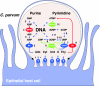
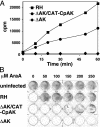
Nucleotide metabolic pathways provide numerous successful targets for antiparasitic chemotherapy, but the human pathogen Cryptosporidium parvum thus far has proved extraordinarily refractory to classical treatments. Given the importance of this protist as an opportunistic pathogen afflicting immunosuppressed individuals, effective treatments are urgently needed. The genome sequence of C. parvum is approaching completion, and we have used this resource to critically assess nucleotide biosynthesis as a target in C. parvum. Genomic analysis indicates that this parasite is entirely dependent on salvage from the host for its purines and pyrimidines. Metabolic pathway reconstruction and experimental validation in the laboratory further suggest that the loss of pyrimidine de novo synthesis is compensated for by possession of three salvage enzymes. Two of these, uridine kinase-uracil phosphoribosyltransferase and thymidine kinase, are unique to C. parvum within the phylum Apicomplexa. Phylogenetic analysis suggests horizontal gene transfer of thymidine kinase from a proteobacterium. We further show that the purine metabolism in C. parvum follows a highly streamlined pathway. Salvage of adenosine provides C. parvum's sole source of purines. This renders the parasite susceptible to inhibition of inosine monophosphate dehydrogenase, the rate-limiting enzyme in the multistep conversion of AMP to GMP. The inosine 5' monophosphate dehydrogenase inhibitors ribavirin and mycophenolic acid, which are already in clinical use, show pronounced anticryptosporidial activity. Taken together, these data help to explain why widely used drugs fail in the treatment of cryptosporidiosis and suggest more promising targets.
Figures

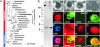

References
-
- MacKenzie, W. R., Schell, W. L., Blair, K. A., Addiss, D. G., Peterson, D. E., Hoxie, N. J., Kazmierczak, J. J. & Davis, J. P. (1995) Clin. Infect. Dis. 21, 57-62. - PubMed
-
- DuPont, H. L., Chappell, C. L., Sterling, C. R., Okhuysen, P. C., Rose, J. B. & Jakubowski, W. (1995) N. Engl. J. Med. 332, 855-859. - PubMed
-
- Mead, J. R. (2002) Drug Resist. Update. 5, 47-57. - PubMed
-
- Striepen, B., He, C. Y., Matrajt, M., Soldati, D. & Roos, D. S. (1998) Mol. Biochem. Parasitol. 92, 325-338. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

