Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice
- PMID: 14973255
- PMCID: PMC6730468
- DOI: 10.1523/JNEUROSCI.4608-03.2004
Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice
Abstract
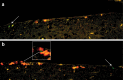
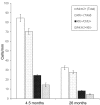
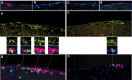
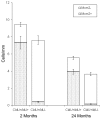
The mammalian brain contains neural stem cells (NSCs) that allow continued neurogenesis throughout the life of the animal. However, neurogenesis is known to decline during aging and, to the extent that neurogenesis is required for normal CNS function, this may contribute to neurodegenerative disease. Decreased neurogenesis could result from loss of NSCs or dysfunction at some later step, and distinguishing these possibilities is important for understanding the cause of the decline. However, because of the inability to distinguish NSCs from their rapidly dividing progeny in situ, it has not been possible to quantitatively assess the NSC populations in young and old animals. In this report we show that the G1 phase-specific expression of the replication factor Mcm2 is a useful marker for detecting slowly cycling putative NSCs in situ and confirm the identity of these cells using both cytosine beta-D-arabinofuranoside (Ara-C) treatment and a double nucleoside analog-labeling technique. The ability to distinguish NSCs from proliferative progenitors has allowed characterization of the expression of several markers including Nestin, Musashi, and GFAP in these different cell types. Furthermore, comparison of the NSC populations in the subventricular zones of young (2-4 months) and old (24-26 months) mice demonstrates an approximately twofold reduction in the older mice. A similar twofold reduction is also observed in the number of neurospheres recovered in culture from old relative to young animals. The reduction in the neural stem cell population documented here is sufficient to account for the reduced level of neurogenesis in old animals.
Figures

References
-
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335. - PubMed
-
- Armstrong RJ, Barker RA (2001) Neurodegeneration: a failure of neuroregeneration? Lancet 358: 1174-1176. - PubMed
-
- Aten JA, Stap J, Hoebe R, Bakker PJ (1994) Application and detection of IdUrd and CldUrd as two independent cell-cycle markers. Methods Cell Biol 41: 317-326. - PubMed
-
- Bickenbach JR (1981) Identification and behavior of label-retaining cells in oral mucosa and skin. J Dent Res 60: 1611-1620. - PubMed
-
- Bickenbach JR, McCutecheon J, Mackenzie IC (1986) Rate of loss of tritiated thymidine label in basal cells in mouse epithelial tissues. Cell Tissue Kinet 19: 325-333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
