LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1
- PMID: 14976552
- PMCID: PMC381014
- DOI: 10.1038/sj.emboj.7600110
LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1
Abstract
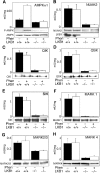
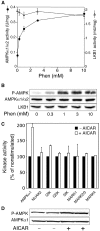
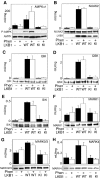
We recently demonstrated that the LKB1 tumour suppressor kinase, in complex with the pseudokinase STRAD and the scaffolding protein MO25, phosphorylates and activates AMP-activated protein kinase (AMPK). A total of 12 human kinases (NUAK1, NUAK2, BRSK1, BRSK2, QIK, QSK, SIK, MARK1, MARK2, MARK3, MARK4 and MELK) are related to AMPK. Here we demonstrate that LKB1 can phosphorylate the T-loop of all the members of this subfamily, apart from MELK, increasing their activity >50-fold. LKB1 catalytic activity and the presence of MO25 and STRAD are required for activation. Mutation of the T-loop Thr phosphorylated by LKB1 to Ala prevented activation, while mutation to glutamate produced active forms of many of the AMPK-related kinases. Activities of endogenous NUAK2, QIK, QSK, SIK, MARK1, MARK2/3 and MARK4 were markedly reduced in LKB1-deficient cells. Neither LKB1 activity nor that of AMPK-related kinases was stimulated by phenformin or AICAR, which activate AMPK. Our results show that LKB1 functions as a master upstream protein kinase, regulating AMPK-related kinases as well as AMPK. Between them, these kinases may mediate the physiological effects of LKB1, including its tumour suppressor function.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

