The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2
- PMID: 14976557
- PMCID: PMC380977
- DOI: 10.1038/sj.emboj.7600122
The Zap1 transcriptional activator also acts as a repressor by binding downstream of the TATA box in ZRT2
Abstract
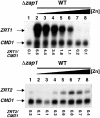
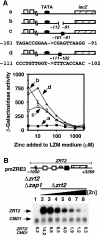
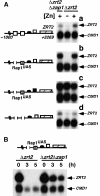
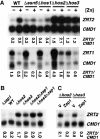
The zinc-responsive transcriptional activator Zap1 regulates the expression of both high- and low-affinity zinc uptake permeases encoded by the ZRT1 and ZRT2 genes. Zap1 mediates this response by binding to zinc-responsive elements (ZREs) located within the promoter regions of each gene. ZRT2 has a remarkably different expression profile in response to zinc compared to ZRT1. While ZRT1 is maximally induced during zinc limitation, ZRT2 is repressed in low zinc but remains induced upon zinc supplementation. In this study, we determined the mechanism underlying this paradoxical Zap1-dependent regulation of ZRT2. We demonstrate that a nonconsensus ZRE (ZRT2 ZRE3), which overlaps with one of the ZRT2 transcriptional start sites, is essential for repression of ZRT2 in low zinc and that Zap1 binds to ZRT2 ZRE3 with a low affinity. The low-affinity ZRE is also essential for the ZRT2 expression profile. These results indicate that the unusual pattern of ZRT2 regulation among Zap1 target genes involves the antagonistic effect of Zap1 binding to a low-affinity ZRE repressor site and high-affinity ZREs required for activation.
Figures



References
-
- Bachhawat N, Ouyang Q, Henry SA (1995) Functional characterization of an inositol-sensitive upstream activation sequence in yeast. J Biol Chem 270: 25087–25095 - PubMed
-
- Banerji J, Rusconi S, Schaffner W (1981) Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell 27: 299–308 - PubMed
-
- Barolo S, Posakony JW (2002) Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev 16: 1167–1181 - PubMed
-
- Bird A, Evans-Galea MV, Blankman E, Zhao H, Luo H, Winge DR, Eide DJ (2000a) Mapping the DNA binding domain of the Zap1 zinc-responsive transcriptional activator. J Biol Chem 275: 16160–16166 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

