Mapping hypoxia-induced bioenergetic rearrangements and metabolic signaling by 18O-assisted 31P NMR and 1H NMR spectroscopy
- PMID: 14977188
- PMCID: PMC2743901
- DOI: 10.1023/b:mcbi.0000009875.30308.7a
Mapping hypoxia-induced bioenergetic rearrangements and metabolic signaling by 18O-assisted 31P NMR and 1H NMR spectroscopy
Abstract
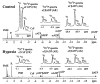
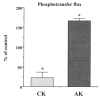
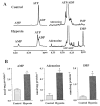
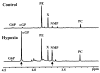
Brief hypoxia or ischemia perturbs energy metabolism inducing paradoxically a stress-tolerant state, yet metabolic signals that trigger cytoprotection remain poorly understood. To evaluate bioenergetic rearrangements, control and hypoxic hearts were analyzed with 18O-assisted 31P NMR and 1H NMR spectroscopy. The 18O-induced isotope shift in the 31P NMR spectrum of CrP, betaADP and betaATP was used to quantify phosphotransfer fluxes through creatine kinase and adenylate kinase. This analysis was supplemented with determination of energetically relevant metabolites in the phosphomonoester (PME) region of 31P NMR spectra, and in both aromatic and aliphatic regions of 1H NMR spectra. In control conditions, creatine kinase was the major phosphotransfer pathway processing high-energy phosphoryls between sites of ATP consumption and ATP production. In hypoxia, creatine kinase flux was dramatically reduced with a compensatory increase in adenylate kinase flux, which supported heart energetics by regenerating and transferring beta- and gamma-phosphoryls of ATP. Activation of adenylate kinase led to a build-up of AMP, IMP and adenosine, molecules involved in cardioprotective signaling. 31P and 1H NMR spectral analysis further revealed NADH and H+ scavenging by alpha-glycerophosphate dehydrogenase (alphaGPDH) and lactate dehydrogenase contributing to maintained glycolysis under hypoxia. Hypoxia-induced accumulation of alpha-glycerophosphate and nucleoside 5'-monophosphates, through alphaGPDH and adenylate kinase reactions, respectively, was mapped within the increased PME signal in the 31P NMR spectrum. Thus, 18O-assisted 31P NMR combined with 1H NMR provide a powerful approach in capturing rearrangements in cardiac bioenergetics, and associated metabolic signaling that underlie the cardiac adaptive response to stress.
Figures
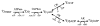

Similar articles
-
Dynamic phosphometabolomic profiling of human tissues and transgenic models by 18O-assisted ³¹P NMR and mass spectrometry.Physiol Genomics. 2012 Apr 2;44(7):386-402. doi: 10.1152/physiolgenomics.00152.2011. Epub 2012 Jan 10. Physiol Genomics. 2012. PMID: 22234996 Free PMC article.
-
Cellular energetics in the preconditioned state: protective role for phosphotransfer reactions captured by 18O-assisted 31P NMR.J Biol Chem. 2001 Nov 30;276(48):44812-9. doi: 10.1074/jbc.M104425200. Epub 2001 Oct 2. J Biol Chem. 2001. PMID: 11583991
-
31P NMR kinetics study of cardiac metabolism under mild hypoxia.J Magn Reson B. 1995 Mar;106(3):212-9. doi: 10.1006/jmrb.1995.1036. J Magn Reson B. 1995. PMID: 7719621
-
MR spectroscopy in heart failure.Front Biosci (Schol Ed). 2011 Jan 1;3(1):331-40. doi: 10.2741/s154. Front Biosci (Schol Ed). 2011. PMID: 21196379 Review.
-
Cardiac MR spectroscopy in the new millennium.Rays. 2001 Jan-Mar;26(1):93-107. Rays. 2001. PMID: 11471351 Review.
Cited by
-
Effect of leucine administration to female rats during pregnancy and lactation on oxidative stress and enzymes activities of phosphoryltransfer network in cerebral cortex and hippocampus of the offspring.Neurochem Res. 2013 Mar;38(3):632-43. doi: 10.1007/s11064-012-0961-4. Epub 2013 Jan 1. Neurochem Res. 2013. PMID: 23277415
-
(31)P NMR correlation maps of (18)O/ (16)O chemical shift isotopic effects for phosphometabolite labeling studies.J Biomol NMR. 2011 Jul;50(3):237-45. doi: 10.1007/s10858-011-9515-3. Epub 2011 May 25. J Biomol NMR. 2011. PMID: 21611840 Free PMC article.
-
Creatine and pyruvate prevent the alterations caused by tyrosine on parameters of oxidative stress and enzyme activities of phosphoryltransfer network in cerebral cortex of Wistar rats.Mol Neurobiol. 2015;51(3):1184-94. doi: 10.1007/s12035-014-8791-9. Epub 2014 Jun 25. Mol Neurobiol. 2015. PMID: 24961569
-
Monitoring ATP hydrolysis and ATPase inhibitor screening using (1)H NMR.Chem Commun (Camb). 2014 Oct 18;50(81):12037-9. doi: 10.1039/c4cc04399e. Chem Commun (Camb). 2014. PMID: 25170530 Free PMC article.
-
Electron spray ionization mass spectrometry and 2D 31P NMR for monitoring 18O/16O isotope exchange and turnover rates of metabolic oligophosphates.Anal Bioanal Chem. 2012 May;403(3):697-706. doi: 10.1007/s00216-012-5899-5. Epub 2012 Mar 18. Anal Bioanal Chem. 2012. PMID: 22427058 Free PMC article.
References
-
- Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–246. - PubMed
-
- Saks VA, Tiivel T, Kay L, Novel-Chate V, Daneshrad Z, Rossi A, Fontaine E, Keriel C, Leverve X, Ventura-Clapier R, Anflous K, Samuel JL, Rappaport L. On the regulation of cellular energetics in health and disease. Mol Cell Biochem. 1996;160–161:195–208. - PubMed
-
- Opie LH. Biochemical changes associated with reversible myocardial ischemia. In: Heyndricks GR, Vatner SF, Wijns W, editors. Clinical Pathophysiology of Myocardial Ischemia. Lippincot-Raven; New York: 1997. pp. 1–19.
-
- Taegtmeyer H, King LM, Jones BE. Energy substrate metabolism, myocardial ischemia, and targets for pharmacotherapy. Am J Cardiol. 1998;82:54K–60K. - PubMed
-
- Piper HM. Energy deficiency, calcium overload or oxidative stress: Possible causes of irreversible ischemic myocardial injury. Klin Wochenschr. 1989;67:465–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
