A model of high-frequency oscillatory potentials in retinal ganglion cells
- PMID: 14977326
- PMCID: PMC3348786
- DOI: 10.1017/s0952523803205010
A model of high-frequency oscillatory potentials in retinal ganglion cells
Abstract
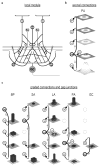
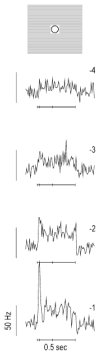
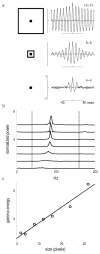
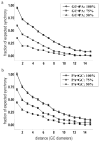
High-frequency oscillatory potentials (HFOPs) have been recorded from ganglion cells in cat, rabbit, frog, and mudpuppy retina and in electroretinograms (ERGs) from humans and other primates. However, the origin of HFOPs is unknown. Based on patterns of tracer coupling, we hypothesized that HFOPs could be generated, in part, by negative feedback from axon-bearing amacrine cells excited via electrical synapses with neighboring ganglion cells. Computer simulations were used to determine whether such axon-mediated feedback was consistent with the experimentally observed properties of HFOPs. (1) Periodic signals are typically absent from ganglion cell PSTHs, in part because the phases of retinal HFOPs vary randomly over time and are only weakly stimulus locked. In the retinal model, this phase variability resulted from the nonlinear properties of axon-mediated feedback in combination with synaptic noise. (2) HFOPs increase as a function of stimulus size up to several times the receptive-field center diameter. In the model, axon-mediated feedback pooled signals over a large retinal area, producing HFOPs that were similarly size dependent. (3) HFOPs are stimulus specific. In the model, gap junctions between neighboring neurons caused contiguous regions to become phase locked, but did not synchronize separate regions. Model-generated HFOPs were consistent with the receptive-field center dynamics and spatial organization of cat alpha cells. HFOPs did not depend qualitatively on the exact value of any model parameter or on the numerical precision of the integration method. We conclude that HFOPs could be mediated, in part, by circuitry consistent with known retinal anatomy.
Figures






Similar articles
-
Stimulus-specific oscillations in a retinal model.IEEE Trans Neural Netw. 2004 Sep;15(5):1083-91. doi: 10.1109/TNN.2004.832722. IEEE Trans Neural Netw. 2004. PMID: 15484885
-
Photoreceptive Ganglion Cells Drive Circuits for Local Inhibition in the Mouse Retina.J Neurosci. 2021 Feb 17;41(7):1489-1504. doi: 10.1523/JNEUROSCI.0674-20.2020. Epub 2021 Jan 4. J Neurosci. 2021. PMID: 33397711 Free PMC article.
-
Gap junctions with amacrine cells provide a feedback pathway for ganglion cells within the retina.Proc Biol Sci. 1998 May 22;265(1399):919-25. doi: 10.1098/rspb.1998.0379. Proc Biol Sci. 1998. PMID: 9633113 Free PMC article.
-
Direction selectivity in the retina: symmetry and asymmetry in structure and function.Nat Rev Neurosci. 2012 Feb 8;13(3):194-208. doi: 10.1038/nrn3165. Nat Rev Neurosci. 2012. PMID: 22314444 Review.
-
Inhibitory Interneurons in the Retina: Types, Circuitry, and Function.Annu Rev Vis Sci. 2017 Sep 15;3:1-24. doi: 10.1146/annurev-vision-102016-061345. Epub 2017 Jun 15. Annu Rev Vis Sci. 2017. PMID: 28617659 Review.
Cited by
-
The photopic negative response of the flash electroretinogram in retinal vein occlusion.Doc Ophthalmol. 2006 Jul;113(1):53-9. doi: 10.1007/s10633-006-9015-z. Epub 2006 Aug 30. Doc Ophthalmol. 2006. PMID: 16944088
-
Contribution of retinal ganglion cells to the mouse electroretinogram.Doc Ophthalmol. 2014 Jun;128(3):155-68. doi: 10.1007/s10633-014-9433-2. Doc Ophthalmol. 2014. PMID: 24659322
-
The photopic negative response of flash ERG in nonproliferative diabetic retinopathy.Doc Ophthalmol. 2008 Sep;117(2):129-35. doi: 10.1007/s10633-008-9114-0. Epub 2008 Jan 23. Doc Ophthalmol. 2008. PMID: 18214565
-
A high frequency resonance in the responses of retinal ganglion cells to rapidly modulated stimuli: a computer model.Vis Neurosci. 2006 Sep-Oct;23(5):779-94. doi: 10.1017/S0952523806230104. Vis Neurosci. 2006. PMID: 17020633 Free PMC article.
-
The temporal structure of transient ON/OFF ganglion cell responses and its relation to intra-retinal processing.J Comput Neurosci. 2006 Oct;21(2):131-51. doi: 10.1007/s10827-006-7863-x. Epub 2006 May 26. J Comput Neurosci. 2006. PMID: 16732489
References
-
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383(6603):815–819. - PubMed
-
- Amthor FR, Grzywacz NM. Synchronous firing is stimulus dependent in directionally selective (DS) and non-DS rabbit ganglion cells. Investigative Ophthalmology & Visual Science. 2001;42(4):S677.
-
- Ariel M, Daw NW, Rader RK. Rhythmicity in rabbit retinal ganglion cell responses. Vision Research. 1983;23(12):1485–1493. - PubMed
-
- Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20(3):527–539. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

