Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors
- PMID: 14977954
- PMCID: PMC356064
- DOI: 10.1128/IAI.72.3.1487-1495.2004
Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors
Abstract
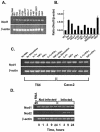
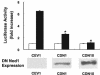
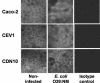
The transcription factor NF-kappaB in human intestinal epithelial cells plays a central role in regulating genes that govern the onset of mucosal inflammatory responses following intestinal microbial infection. Nod1 is a cytosolic pattern recognition receptor in mammalian cells that senses components of microbial peptidoglycans and signals the activation of NF-kappaB. The aim of these studies was to assess the functional importance of Nod1 in activating NF-kappaB and NF-kappaB proinflammatory target genes in human intestinal epithelium. Human colon epithelial cells that constitutively express Nod1 were used as a model intestinal epithelium. These cells do not signal through Toll-like receptor 4 (TLR4) or respond to bacterial lipopolysaccharide, but they express functional TLR5 and interleukin 1 (IL-1) receptors that signal the activation of NF-kappaB in response to bacterial flagellin or IL-1 stimulation. Stable expression of dominant negative (DN) Nod1 in colon epithelial cells prevented IkappaB kinase and NF-kappaB activation in response to infection with enteroinvasive Escherichia coli. In contrast, DN Nod1 did not eliminate IL-1 or flagellin-stimulated NF-kappaB activation. Inhibition of NF-kappaB was accompanied by inhibition of NF-kappaB target genes that provide signals for the mucosal influx of neutrophils during intestinal infection. We conclude that signaling through Nod1 is required for activating NF-kappaB in human intestinal epithelial cells infected with gram-negative enteric bacteria that can bypass TLR activation. Signaling through Nod1 provides the intestinal epithelium with a backup mechanism for rapidly activating innate immunity during infection with a group of highly invasive pathogenic gram-negative bacteria.
Figures




References
-
- Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. - PubMed
-
- Akhtar, M., J. L. Watson, A. Nazli, and D. M. McKay. 2003. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-κB-independent pathway. FASEB J. 17:1319-1321. - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 4:635-648. - PubMed
-
- Berin, M. C., M. B. Dwinell, L. Eckmann, and M. F. Kagnoff. 2001. Production of MDC/CCL22 by human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G1217-G1226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

