The Burkholderia cepacia epidemic strain marker is part of a novel genomic island encoding both virulence and metabolism-associated genes in Burkholderia cenocepacia
- PMID: 14977960
- PMCID: PMC356040
- DOI: 10.1128/IAI.72.3.1537-1547.2004
The Burkholderia cepacia epidemic strain marker is part of a novel genomic island encoding both virulence and metabolism-associated genes in Burkholderia cenocepacia
Abstract
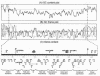
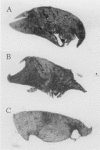
The Burkholderia cepacia epidemic strain marker (BCESM) is a useful epidemiological marker for virulent B. cenocepacia strains that infect patients with cystic fibrosis. However, there was no evidence that the original marker, identified by random amplified polymorphic DNA fingerprinting, contributed to pathogenicity. Here we demonstrate that the BCESM is part of a novel genomic island encoding genes linked to both virulence and metabolism. The BCESM was present on a 31.7-kb low-GC-content island that encoded 35 predicted coding sequences (CDSs): an N-acyl homoserine lactone (AHL) synthase gene (cciI) and corresponding transcriptional regulator (cciR), representing the first time cell signaling genes have been found on a genomic island; fatty acid biosynthesis genes; an IS66 family transposase; transcriptional regulator CDSs; amino acid metabolism genes; and a group of hypothetical genes. Mutagenesis of the AHL synthase, amidase (amiI), and porin (opcI) genes on the island was carried out. Testing of the isogenic mutants in a rat model of chronic lung infection demonstrated that the amidase played a role in persistence, while the AHL synthase and porin were both involved in virulence. The island, designated the B. cenocepacia island (cci), is the first genomic island to be defined in the B. cepacia complex and its discovery validates the original epidemiological correlation of the BCESM with virulent CF strains. The features of the cci, which overlap both pathogenicity and metabolism, expand the concept of bacterial pathogenicity islands and illustrate the diversity of accessory functions that can be acquired by lateral gene transfer in bacteria.
Figures



References
-
- Achouak, W., T. Heulin, and J. M. Pages. 2001. Multiple facets of bacterial porins. FEMS Microbiol. Lett. 199:1-7. - PubMed
-
- Aris, R. M., J. C. Routh, J. J. LiPuma, D. G. Heath, and P. H. Gilligan. 2001. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am. J. Respir. Crit. Care Med. 164:2102-2106. - PubMed
-
- Atkinson, S., J. P. Throup, G. S. Stewart, and P. Williams. 1999. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267-1277. - PubMed
-
- Botelho, B. A., S. Y. Bando, L. R. Trabulsi, and C. A. Moreira-Filho. 2003. Identification of EPEC and non-EPEC serotypes in the EPEC O serogroups by PCR-RFLP analysis of the fliC gene. J. Microbiol. Methods 54:87-93. - PubMed
-
- Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

