OspB antibody prevents Borrelia burgdorferi colonization of Ixodes scapularis
- PMID: 14977984
- PMCID: PMC356050
- DOI: 10.1128/IAI.72.3.1755-1759.2004
OspB antibody prevents Borrelia burgdorferi colonization of Ixodes scapularis
Abstract
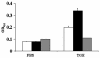
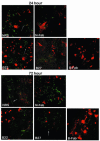
Borrelia burgdorferi outer surface protein OspB is expressed by spirochetes in the Ixodes scapularis gut. ospB is transcribed from a bicistronic operon with ospA, a known spirochete adhesion gene in the tick gut. Here we examine whether OspB also has a specific function in ticks. OspB specifically binds to a protein or protein complex within the tick gut. We also assessed whether selected nonborreliacidal OspB antibodies or F(ab)(2) fragments interfere with B. burgdorferi-tick attachment in vivo. We examined engorged ticks that fed on B. burgdorferi N40-infected scid mice that had been treated with OspB F(ab)(2) fragments. Control F(ab)(2) fragments did not interfere with B. burgdorferi colonization of the tick gut, whereas OspB F(ab)(2) fragments significantly inhibited the attachment of spirochetes to the tick gut. These studies show that nonbactericidal OspB antibodies interfere with B. burgdorferi colonization of I. scapularis, highlighting a specific role for OspB in spirochete- arthropod interactions and suggesting new antibody-mediated strategies for interfering with B. burgdorferi transmission.
Figures


References
-
- Brunet, L. R., A. Spielman, E. Fikrig, and S. R. Telford, 3rd. 1997. Heterogeneity of Lyme disease spirochaetes within individual vector ticks. Res. Microbiol. 148:437-445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

