Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A
- PMID: 14977987
- PMCID: PMC356054
- DOI: 10.1128/IAI.72.3.1775-1785.2004
Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A
Abstract
The gonococcal transferrin receptor is composed of two distinct proteins, TbpA and TbpB. TbpA is a member of the TonB-dependent family of integral outer membrane transporters, while TbpB is lipid modified and thought to be peripherally surface exposed. We previously proposed a hypothetical topology model for gonococcal TbpA that was based upon computer predictions and similarity with other TonB-dependent transporters for which crystal structures have been determined. In the present study, the hemagglutinin epitope was inserted into TbpA to probe the surface topology of this protein and secondarily to test the functional impacts of site-specific mutagenesis. Twelve epitope insertion mutants were constructed, five of which allowed us to confirm the surface exposure of loops 2, 3, 5, 7, and 10. In contrast to the predictions set forth by the hypothetical model, insertion into the plug region resulted in an epitope that was surface accessible, while epitope insertions into two putative loops (9 and 11) were not surface accessible. Insertions into putative loop 3 and beta strand 9 abolished transferrin binding and utilization, and the plug insertion mutant exhibited decreased transferrin-binding affinity concomitant with an inability to utilize it. Insertion into putative beta strand 16 generated a mutant that was able to bind transferrin normally but that was unable to mediate utilization. Mutants with insertions into putative loops 2, 9, and 11 maintained wild-type binding affinity but could utilize only transferrin in the presence of TbpB. This is the first demonstration of the ability of TbpB to compensate for a mutation in TbpA.
Figures






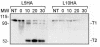
Similar articles
-
Identification of TbpA residues required for transferrin-iron utilization by Neisseria gonorrhoeae.Infect Immun. 2008 May;76(5):1960-9. doi: 10.1128/IAI.00020-08. Epub 2008 Mar 17. Infect Immun. 2008. PMID: 18347046 Free PMC article.
-
Identification of transferrin-binding domains in TbpB expressed by Neisseria gonorrhoeae.Infect Immun. 2007 Jul;75(7):3220-32. doi: 10.1128/IAI.00072-07. Epub 2007 Apr 16. Infect Immun. 2007. PMID: 17438025 Free PMC article.
-
Evidence of Fe3+ interaction with the plug domain of the outer membrane transferrin receptor protein of Neisseria gonorrhoeae: implications for Fe transport.Metallomics. 2012 Apr;4(4):361-72. doi: 10.1039/c2mt20037f. Epub 2012 Mar 8. Metallomics. 2012. PMID: 22399131 Free PMC article.
-
Iron acquisition through the bacterial transferrin receptor.Crit Rev Biochem Mol Biol. 2017 Jun;52(3):314-326. doi: 10.1080/10409238.2017.1293606. Epub 2017 Mar 1. Crit Rev Biochem Mol Biol. 2017. PMID: 28276700 Review.
-
Transferrin-iron uptake by Gram-negative bacteria.Front Biosci. 2003 May 1;8:d836-47. doi: 10.2741/1076. Front Biosci. 2003. PMID: 12700102 Review.
Cited by
-
Identification of amino acid residues involved in heme binding and hemoprotein utilization in the Porphyromonas gingivalis heme receptor HmuR.Infect Immun. 2006 Feb;74(2):1222-32. doi: 10.1128/IAI.74.2.1222-1232.2006. Infect Immun. 2006. PMID: 16428772 Free PMC article.
-
Structural basis for iron piracy by pathogenic Neisseria.Nature. 2012 Feb 12;483(7387):53-8. doi: 10.1038/nature10823. Nature. 2012. PMID: 22327295 Free PMC article.
-
Point Mutations in TbpA Abrogate Human Transferrin Binding in Neisseria gonorrhoeae.Infect Immun. 2022 Nov 17;90(11):e0041422. doi: 10.1128/iai.00414-22. Epub 2022 Nov 2. Infect Immun. 2022. PMID: 36321833 Free PMC article.
-
Conserved regions of gonococcal TbpB are critical for surface exposure and transferrin iron utilization.Infect Immun. 2013 Sep;81(9):3442-50. doi: 10.1128/IAI.00280-13. Epub 2013 Jul 8. Infect Immun. 2013. PMID: 23836816 Free PMC article.
-
Identification of TbpA residues required for transferrin-iron utilization by Neisseria gonorrhoeae.Infect Immun. 2008 May;76(5):1960-9. doi: 10.1128/IAI.00020-08. Epub 2008 Mar 17. Infect Immun. 2008. PMID: 18347046 Free PMC article.
References
-
- Armstrong, S. K., and M. A. McIntosh. 1995. Epitope insertions define functional and topological features of the Escherichia coli ferric enterobactin receptor. J. Biol. Chem. 270:2483-2488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

