Characterization of cytolethal distending toxin genes and expression in shiga toxin-producing Escherichia coli strains of non-O157 serogroups
- PMID: 14977993
- PMCID: PMC356029
- DOI: 10.1128/IAI.72.3.1812-1816.2004
Characterization of cytolethal distending toxin genes and expression in shiga toxin-producing Escherichia coli strains of non-O157 serogroups
Abstract
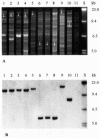
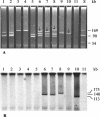
We identified cytolethal distending toxin and its gene (cdt) in 17 of 340 non-O157 Shiga toxin-producing Escherichia coli (STEC) strains (serotypes O73:H18, O91:H21, O113:H21, and O153:H18), all of which were eae negative. cdt is either chromosomal and homologous to cdt-V (serotypes O73:H18, O91:H21, and O113:H21) or plasmidborne and identical to cdt-III (serotype O153:H18). Among eae-negative STEC, cdt was associated with disease (P = 0.003).
Figures

References
-
- Ansaruzzaman, M., M. J. Albert, S. Nahar, R. Byun, M. Katouli, I. Kuhn, and R. Mölby. 2000. Clonal groups of enteropathogenic Escherichia coli isolated in case-control studies of diarrhoea in Bangladesh. J. Med. Microbiol. 49:177-185. - PubMed
-
- Cortes-Bratti, X., T. Frisan, and M. Thelestam. 2001. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon 39:1729-1736. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

