Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry
- PMID: 14978168
- PMCID: PMC2587250
- DOI: 10.1097/01.asn.0000113553.62380.f5
Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry
Abstract
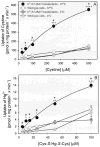
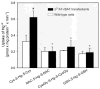
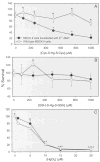
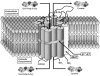
Humans and other mammals continue to be exposed to various forms of mercury in the environment. The kidneys, specifically the epithelial cells lining the proximal tubules, are the primary targets where mercuric ions accumulate and exert their toxic effects. Although the actual mechanisms involved in the transport of mercuric ions along the proximal tubule have not been defined, current evidence implicates mercuric conjugates of cysteine, primarily 2-amino-3-(2-amino-2-carboxyethylsulfanylmercuricsulfanyl)propionic acid (Cys-S-Hg-S-Cys), as the most likely transportable species of inorganic mercury (Hg(2+)). Because Cys-S-Hg-S-Cys and the amino acid cystine (Cys-S-S-Cys) are structurally similar, it was hypothesized that Cys-S-Hg-S-Cys might act as a molecular mimic of cystine at one or more of the amino acid transporters involved in the luminal absorption of this amino acid. One such candidate is the Na(+)-independent heterodimeric transporter system b(0,+). Therefore, the transport of Cys-S-Hg-S-Cys and cystine was studied in MDCK II cells that were or were not stably transfected with b(0,+)AT-rBAT. Transport of Cys-S-Hg-S-Cys and cystine across the luminal plasma membrane was similar in the transfected cells, indicating that Cys-S-Hg-S-Cys can behave as a molecular mimic of cystine at the site of system b(0,+). Moreover, only the b(0,+)AT-rBAT transfectants became selectively intoxicated during exposure to Cys-S-Hg-S-Cys. These findings indicate that system b(0,+) likely contributes to the nephropathy induced by Hg(2+) in vivo. These data represent the first direct molecular evidence for the participation of a specific transporter in the luminal uptake of a large divalent metal cation in proximal tubular cells.
Figures







References
-
- Zalups RK, Lash LH. Advances in understanding the renal transport and toxicity of mercury. J Toxicol Environ Health. 1994;42:1–44. - PubMed
-
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52:113–143. - PubMed
-
- Berndt WO, Baggett JMcC, Blacker A, Houser M. Renal glutathione and mercury uptake by kidney. Fundam Appl Toxicol. 1985;5:832–839. - PubMed
-
- Tanaka T, Naganuma A, Imura N. Role of γ-glutamyltranspeptidase in renal uptake and toxicity of inorganic mercury in mice. Toxicology. 1990;60:187–198. - PubMed
-
- Tanaka-Kagawa T, Naganuma A, Imura N. Tubular secretion and reabsorption of mercury compounds in mouse kidney. J Pharmacol Exp Ther. 1993;264:776–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

