Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro
- PMID: 14978199
- PMCID: PMC1665051
- DOI: 10.1113/jphysiol.2003.055772
Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro
Abstract
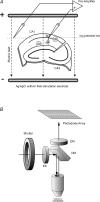
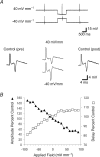
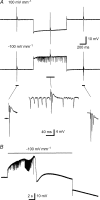
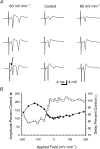
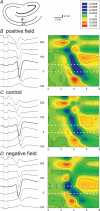
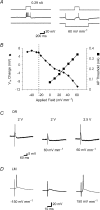
The effects of uniform steady state (DC) extracellular electric fields on neuronal excitability were characterized in rat hippocampal slices using field, intracellular and voltage-sensitive dye recordings. Small electric fields (</40/ mV mm(-1)), applied parallel to the somato-dendritic axis, induced polarization of CA1 pyramidal cells; the relationship between applied field and induced polarization was linear (0.12 +/- 0.05 mV per mV mm(-1) average sensitivity at the soma). The peak amplitude and time constant (15-70 ms) of membrane polarization varied along the axis of neurons with the maximal polarization observed at the tips of basal and apical dendrites. The polarization was biphasic in the mid-apical dendrites; there was a time-dependent shift in the polarity reversal site. DC fields altered the thresholds of action potentials evoked by orthodromic stimulation, and shifted their initiation site along the apical dendrites. Large electric fields could trigger neuronal firing and epileptiform activity, and induce long-term (>1 s) changes in neuronal excitability. Electric fields perpendicular to the apical-dendritic axis did not induce somatic polarization, but did modulate orthodromic responses, indicating an effect on afferents. These results demonstrate that DC fields can modulate neuronal excitability in a time-dependent manner, with no clear threshold, as a result of interactions between neuronal compartments, the non-linear properties of the cell membrane, and effects on afferents.
Figures

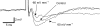


References
-
- Andreasen M, Nedergaard S. Dendritic electrogenesis in rat hippocampal CA1 pyramidal neurons: functional aspects of Na+ and Ca2+ currents in apical dendrites. Hippocampus. 1996;6:79–95. - PubMed
-
- Bawin SM, Sheppard AR, Mahoney MD, Abu-Assal M, Adey WR. Comparison between the effects of extracellular direct and sinusoidal currents on excitability in hippocampal slices. Brain Res. 1986;362:350–354. - PubMed
-
- Bawin SM, Sheppard AR, Mahoney MD, Adey WR. Influences of sinusoidal electric fields on excitability in the rat hippocampal slice. Brain Res. 1984;323:227–237. - PubMed
-
- Benabid AL, Koudsie A, Pollak P, Kahane P, Chabardes S, Hirsch E, Marescaux C, Benazzouz A. Future prospects of brain stimulation. Neurol Res. 2000;22:237–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

