P2Y purinergic receptor regulation of CFTR chloride channels in mouse cardiac myocytes
- PMID: 14978203
- PMCID: PMC1664988
- DOI: 10.1113/jphysiol.2003.059881
P2Y purinergic receptor regulation of CFTR chloride channels in mouse cardiac myocytes
Abstract
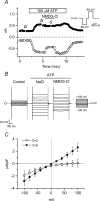
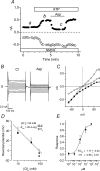
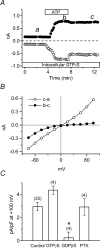
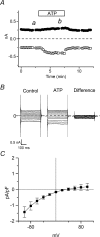
The intracellular signalling pathways and molecular mechanisms responsible for P2-purinoceptor-mediated chloride (Cl(-)) currents (I(Cl,ATP)) were studied in mouse ventricular myocytes. In standard NaCl-containing extracellular solutions, extracellular ATP (100 microm) activated two different currents, I(Cl,ATP) with a linear I-V relationship in symmetrical Cl(-) solutions, and an inwardly rectifying cation conductance (cationic I(ATP)). Cationic I(ATP) was selectively inhibited by Gd(3+) and Zn(2+), or by replacement of extracellular NaCl by NMDG; I(Cl,ATP) was Cl(-) selective, and inhibited by replacement of extracellular Cl(-) by Asp(-); both currents were prevented by suramin or DIDS pretreatment. In GTPgammaS-loaded cells, I(Cl,ATP) was irreversibly activated by ATP, but cationic I(ATP) was still regulated reversibly. GDPbetaS prevented activation of the I(Cl,ATP,) even though pertussis toxin pretreatment did not modulate I(Cl,ATP). These results suggest that activation of I(Cl,ATP) occurs via a G-protein coupled P2Y purinergic receptor. The I(Cl,ATP) persistently activated by GTPgammaS, was inhibited by glibenclamide but not by DIDS, thus exhibiting known pharmacological properties of cystic fibrosis transmembrane conductance regulator (CFTR) Cl(-) channels. In ventricular cells of cftr(-/-) mice, extracellular ATP activated cationic I(ATP), but failed to activate any detectable I(Cl,ATP). These results provide compelling evidence that activation of CFTR Cl(-) channels in mouse heart are coupled to G-protein coupled P2Y purinergic receptors.
Figures

Similar articles
-
Regulation of extracellular UTP-activated Cl- current by P2Y-PLC-PKC signaling and ATP hydrolysis in mouse ventricular myocytes.J Physiol Sci. 2007 Apr;57(2):85-94. doi: 10.2170/physiolsci.RP011406. Epub 2007 Feb 11. J Physiol Sci. 2007. PMID: 17291397
-
Lack of involvement of G proteins in the activation of cardiac CFTR Cl- current by genistein.Pflugers Arch. 1999 May;437(6):796-803. doi: 10.1007/s004240050848. Pflugers Arch. 1999. PMID: 10370056
-
Purinoceptor-coupled Cl- channels in mouse heart: a novel, alternative pathway for CFTR regulation.J Physiol. 1999 Nov 15;521 Pt 1(Pt 1):43-56. doi: 10.1111/j.1469-7793.1999.00043.x. J Physiol. 1999. PMID: 10562333 Free PMC article.
-
Selective activation of cystic fibrosis transmembrane conductance regulator Cl- and HCO3- conductances.JOP. 2001 Jul;2(4 Suppl):212-8. JOP. 2001. PMID: 11875262 Review.
-
The cystic fibrosis transmembrane conductance regulator and ATP.Curr Opin Cell Biol. 1997 Aug;9(4):547-52. doi: 10.1016/s0955-0674(97)80032-4. Curr Opin Cell Biol. 1997. PMID: 9261051 Review.
Cited by
-
In vivo characterization of the role of tissue-specific translation elongation factor 1A2 in protein synthesis reveals insights into muscle atrophy.FEBS J. 2013 Dec;280(24):6528-40. doi: 10.1111/febs.12554. FEBS J. 2013. PMID: 24460877 Free PMC article.
-
Arrhythmia and sudden death associated with elevated cardiac chloride channel activity.J Cell Mol Med. 2011 Nov;15(11):2307-16. doi: 10.1111/j.1582-4934.2010.01243.x. J Cell Mol Med. 2011. PMID: 21155978 Free PMC article.
-
Interaction of P2 purinergic receptors with cellular macromolecules.Naunyn Schmiedebergs Arch Pharmacol. 2008 Mar;377(1):1-33. doi: 10.1007/s00210-007-0222-2. Epub 2007 Dec 19. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18273661 Free PMC article. Review.
-
Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile.Gastroenterology. 2007 Nov;133(5):1592-602. doi: 10.1053/j.gastro.2007.08.020. Epub 2007 Aug 14. Gastroenterology. 2007. PMID: 17916355 Free PMC article.
-
Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart.J Physiol. 2009 May 15;587(Pt 10):2163-77. doi: 10.1113/jphysiol.2008.165860. Epub 2009 Jan 26. J Physiol. 2009. PMID: 19171656 Free PMC article. Review.
References
-
- Devuyst O, Guggino WB. Chloride channels in the kidney: lessons learned from knockout animals. Am J Physiol Renal Physiol. 2002;283:F1176–F1191. - PubMed
-
- Dubyak GR. Knock-out mice reveal tissue-specific roles of P2Y receptor subtypes in different epithelia. Mol Pharmacol. 2003;63:773–776. - PubMed
-
- Ehara T, Ishihara K. Anion channels activated by adrenaline in cardiac myocytes. Nature. 1990;347:284–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

