Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs
- PMID: 14978216
- PMCID: PMC404016
- DOI: 10.1091/mbc.e03-07-0493
Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs
Abstract
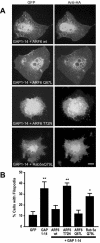
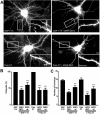
Although neuronal axons and dendrites with their associated filopodia and spines exhibit a profound cell polarity, the mechanism by which they develop is largely unknown. Here, we demonstrate that specific palmitoylated protein motifs, characterized by two adjacent cysteines and nearby basic residues, are sufficient to induce filopodial extensions in heterologous cells and to increase the number of filopodia and the branching of dendrites and axons in neurons. Such motifs are present at the N-terminus of GAP-43 and the C-terminus of paralemmin, two neuronal proteins implicated in cytoskeletal organization and filopodial outgrowth. Filopodia induction is blocked by mutations of the palmitoylated sites or by treatment with 2-bromopalmitate, an agent that inhibits protein palmitoylation. Moreover, overexpression of a constitutively active form of ARF6, a GTPase that regulates membrane cycling and dendritic branching reversed the effects of the acylated protein motifs. Filopodia induction by the specific palmitoylated motifs was also reduced upon overexpression of a dominant negative form of the GTPase cdc42. These results demonstrate that select dually lipidated protein motifs trigger changes in the development and growth of neuronal processes.
Figures






References
-
- Aarts, L.H., Verkade, P., van Dalen, J.J., van Rozen, A.J., Gispen, W.H., Schrama, L.H., and Schotman, P. (1999). B-50/GAP-43 potentiates cytoskeletal reorganization in raft domains. Mol. Cell Neurosci. 14, 85–97. - PubMed
-
- Anderson, S.A., Classey, J.D., Conde, F., Lund, J.S., and Lewis, D.A. (1995). Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience 67, 7–22. - PubMed
-
- Arni, S., Keilbaugh, S.A., Ostermeyer, A.G., and Brown, D.A. (1998). Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J. Biol. Chem. 273, 28478–28485. - PubMed
-
- Benowitz, L.I., and Routtenberg, A. (1997). GAP-43, an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20, 84–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

