A role for sorting nexin 2 in epidermal growth factor receptor down-regulation: evidence for distinct functions of sorting nexin 1 and 2 in protein trafficking
- PMID: 14978220
- PMCID: PMC404011
- DOI: 10.1091/mbc.e03-09-0711
A role for sorting nexin 2 in epidermal growth factor receptor down-regulation: evidence for distinct functions of sorting nexin 1 and 2 in protein trafficking
Abstract
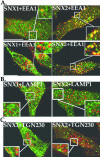
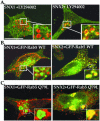
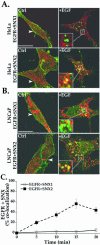
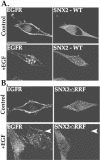
Sorting nexin 1 (SNX1) and SNX2, homologues of the yeast vacuolar protein-sorting (Vps)5p, contain a phospholipid-binding motif termed the phox homology (PX) domain and a carboxyl terminal coiled-coil region. A role for SNX1 in trafficking of cell surface receptors from endosomes to lysosomes has been proposed; however, the function of SNX2 remains unknown. Toward understanding the function of SNX2, we first examined the distribution of endogenous protein in HeLa cells. We show that SNX2 resides primarily in early endosomes, whereas SNX1 is found partially in early endosomes and in tubulovesicular-like structures distributed throughout the cytoplasm. We also demonstrate that SNX1 interacts with the mammalian retromer complex through its amino terminal domain, whereas SNX2 does not. Moreover, activated endogenous epidermal growth factor receptor (EGFR) colocalizes markedly with SNX2-positive endosomes, but minimally with SNX1-containing vesicles. To assess SNX2 function, we examined the effect of a PX domain-mutated SNX2 that is defective in vesicle localization on EGFR trafficking. Mutant SNX2 markedly inhibited agonist-induced EGFR degradation, whereas internalization remained intact. In contrast, SNX1 PX domain mutants failed to effect EGFR degradation, whereas a SNX1 deletion mutant significantly inhibited receptor down-regulation. Interestingly, knockdown of SNX1 and SNX2 expression by RNA interference failed to alter agonist-induced EGFR down-regulation. Together, these findings suggest that both SNX1 and SNX2 are involved in regulating lysosomal sorting of internalized EGFR, but neither protein is essential for this process. These studies are the first to demonstrate a function for SNX2 in protein trafficking.
Figures







References
-
- Bravo, J., et al. (2001). The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol. Cell. 8, 829–839. - PubMed
-
- Carter, R.E., and Sorkin, A. (1998). Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J. Biol. Chem. 273, 35000–35007. - PubMed
-
- Chin, L.S., Raynor, M.C., Wei, X., Chen, H.Q., and Li, L. (2001). Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J. Biol. Chem. 276, 7069–7078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

