Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease
- PMID: 14978262
- PMCID: PMC365771
- DOI: 10.1073/pnas.0400243101
Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease
Abstract
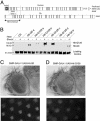
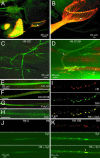
Huntington's disease is an autosomal dominant neurodegenerative disorder caused by expansion of a polyglutamine tract in the huntingtin protein that results in intracellular aggregate formation and neurodegeneration. Pathways leading from polyglutamine tract expansion to disease pathogenesis remain obscure. To elucidate how polyglutamine expansion causes neuronal dysfunction, we generated Drosophila transgenic strains expressing human huntingtin cDNAs encoding pathogenic (Htt-Q128) or nonpathogenic proteins (Htt-Q0). Whereas expression of Htt-Q0 has no discernible effect on behavior, lifespan, or neuronal morphology, pan-neuronal expression of Htt-Q128 leads to progressive loss of motor coordination, decreased lifespan, and time-dependent formation of huntingtin aggregates specifically in the cytoplasm and neurites. Huntingtin aggregates sequester other expanded polyglutamine proteins in the cytoplasm and lead to disruption of axonal transport and accumulation of aggregates at synapses. In contrast, Drosophila expressing an expanded polyglutamine tract alone, or an expanded polyglutamine tract in the context of the spinocerebellar ataxia type 3 protein, display only nuclear aggregates and do not disrupt axonal trafficking. Our findings indicate that nonnuclear events induced by cytoplasmic huntingtin aggregation play a central role in the progressive neurodegeneration observed in Huntington's disease.
Figures



References
-
- The Huntington's Disease Collaborative Research Group (1993) Cell 72, 971-983. - PubMed
-
- Scherzinger, E., Lurz, R., Turmaine, M., Mangiarini, L., Hollenbach, B., Hasenbank, R., Bates, G. P., Davies, S. W., Lehrach, H. & Wanker, E. E. (1997) Cell 90, 549-558. - PubMed
-
- Kopito, R. R. (2000) Trends Cell Biol. 10, 524-530. - PubMed
-
- DiFiglia, M., Sapp, E., Chase, K. O., Davies, S. W., Bates, G. P., Vonsattel, J. P. & Aronin, N. (1997) Science 277, 1990-1993. - PubMed
-
- Davies, S. W., Turmaine, M., Cozens, B. A., DiFiglia, M., Sharp, A. H., Ross, C. A., Scherzinger, E., Wanker, E. E., Mangiarini, L. & Bates, G. P. (1997) Cell 90, 537-548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

