Laser light-scattering evidence for an altered association of beta B1-crystallin deamidated in the connecting peptide
- PMID: 14978307
- PMCID: PMC2286738
- DOI: 10.1110/ps.03427504
Laser light-scattering evidence for an altered association of beta B1-crystallin deamidated in the connecting peptide
Abstract
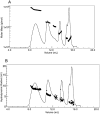
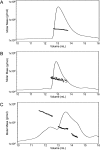
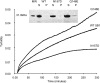
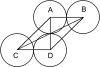
Deamidation is a prevalent modification of crystallin proteins in the vertebrate lens. The effect of specific sites of deamidation on crystallin stability in vivo is not known. Using mass spectrometry, a previously unreported deamidation in beta B1-crystallin was identified at Gln146. Another deamidation was investigated at Asn157. It was determined that whole soluble beta B1 contained 13%-17% deamidation at Gln146 and Asn157. Static and quasi-elastic laser light scattering, circular dichroism, and heat aggregation studies were used to explore the structure and associative properties of recombinantly expressed wild-type (wt) beta B1 and the deamidated beta B1 mutants, Q146E and N157D. Dimer formation occurred for wt beta B1, Q146E, and N157D in a concentration-dependent manner, but only Q146E showed formation of higher ordered oligomers at the concentrations studied. Deamidation at Gln146, but not Asn157, led to an increased tendency of beta B1 to aggregate upon heating. We conclude that deamidation creates unique effects depending upon where the deamidation is introduced in the crystallin structure.
Figures



References
-
- Bateman, O.A., Lubsen, N.H., and Slingsby, C. 2001. Association behaviour of human βB1-crystallin and its truncated forms. Exp. Eye Res. 73 321–331. - PubMed
-
- Bax, B., Lapatto, R., Nalini, V., Driessen, H., Lindley, P.F., Mahadevan, D., Blundell, T.L., and Slingsby, C. 1990. X-ray analysis of β B2-crystallin and evolution of oligomeric lens proteins. Nature 347 776–780. - PubMed
-
- Bindels, J.G., Koppers, A., and Hoenders, H.J. 1981. Structural aspects of bovine β-crystallins: Physical characterization including dissociation–association behavior. Exp. Eye Res. 33 333–343. - PubMed
-
- Blundell, T., Lindley, P., Miller, L., Moss, D., Slingsby, C., Tickle, I., Turnell, B., and Wistow, G. 1981. The molecular structure and stability of the eye lens: X-ray analysis of γ-crystallin II. Nature 289 771–777. - PubMed
-
- Cantor, C.R. and Schimmel, P.R. 1980. Biophysical chemistry. W.H. Freeman, San Francisco.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

