A short synthetic peptide inhibits signal transduction, migration and angiogenesis mediated by Tie2 receptor
- PMID: 14978510
- PMCID: PMC1299011
- DOI: 10.1038/sj.embor.7400100
A short synthetic peptide inhibits signal transduction, migration and angiogenesis mediated by Tie2 receptor
Abstract
Tie2, an endothelial cell-specific receptor kinase, has an important role in tumour angiogenesis. In an attempt to identify peptides that specifically interact with and block the Tie2 pathway, a phage-displayed peptide library was screened on a recombinant Tie2 receptor. One peptide, NLLMAAS, completely abolished the binding to Tie2 of both angiopoietin 2 and angiopoietin 1 (Ang1). We further show that NLLMAAS specifically suppresses both Ang1-induced ERK activity and migration in human umbilical endothelial cells. Moreover, in vivo, this peptide inhibits angiogenesis in the chick chorioallantoic membrane assay. NLLMAAS is the first peptide described to interact with Tie2. Our results demonstrate that it is an efficient and specific antagonist of the binding of Tie2 ligands, and suggest that this peptide or its derivates may have potential applications in the treatment of angiogenesis diseases. It also represents a potent tool to dissect the molecular mechanisms involved in the Tie2 pathway.
Figures
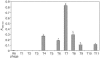


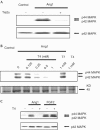

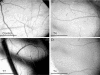
References
-
- Appeldoorn CC, Molenaar TJ, Bonnefoy A, van Leeuwen SH, Vandervoort PA, Hoylaerts MF, van Berkel TJ, Biessen EA ( 2003) Rational optimization of a short human P-selectin-binding peptide leads to nanomolar affinity antagonists. J Biol Chem 278: 10201–10207 - PubMed
-
- Carmeliet P ( 2003) Angiogenesis in health and disease. Nat Med 9: 653–660 - PubMed
-
- Cwirla SE et al. ( 1997) Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276: 1696–1699 - PubMed
-
- Davis S et al. ( 1996) Isolation of angiopoietin-1 a ligand for the Tie-2 receptor, by secretion-trap expression cloning. Cell 87: 1161–1169 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

