Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action
- PMID: 14979919
- PMCID: PMC400651
- DOI: 10.1186/bcr752
Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action
Abstract
Introduction: The oncoprotective role of food-derived polyphenol antioxidants has been described but the implicated mechanisms are not yet clear. In addition to polyphenols, phenolic acids, found at high concentrations in a number of plants, possess antioxidant action. The main phenolic acids found in foods are derivatives of 4-hydroxybenzoic acid and 4-hydroxycinnamic acid.
Methods: This work concentrates on the antiproliferative action of caffeic acid, syringic acid, sinapic acid, protocatechuic acid, ferulic acid and 3,4-dihydroxy-phenylacetic acid (PAA) on T47D human breast cancer cells, testing their antioxidant activity and a number of possible mechanisms involved (interaction with membrane and intracellular receptors, nitric oxide production).
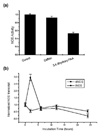
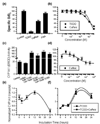
Results: The tested compounds showed a time-dependent and dose-dependent inhibitory effect on cell growth with the following potency: caffeic acid > ferulic acid = protocatechuic acid = PAA > sinapic acid = syringic acid. Caffeic acid and PAA were chosen for further analysis. The antioxidative activity of these phenolic acids in T47D cells does not coincide with their inhibitory effect on tumoral proliferation. No interaction was found with steroid and adrenergic receptors. PAA induced an inhibition of nitric oxide synthase, while caffeic acid competes for binding and results in an inhibition of aryl hydrocarbon receptor-induced CYP1A1 enzyme. Both agents induce apoptosis via the Fas/FasL system.
Conclusions: Phenolic acids exert a direct antiproliferative action, evident at low concentrations, comparable with those found in biological fluids after ingestion of foods rich in phenolic acids. Furthermore, the direct interaction with the aryl hydrocarbon receptor, the nitric oxide synthase inhibition and their pro-apoptotic effect provide some insights into their biological mode of action.
Figures

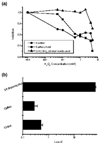
 , in which y is the observed ratio of the cell number in the presence/absence of the phenolic acid, y0 is the maximal observed decrease of cell proliferation (plateau), A1 is 1 - y0, x0 is the initial time of observation (= 0), and x is the time (in days) of the y observation (abscissa of the y observation). The equation after transformation is rearranged as ln [(y - y0) / (1 - y0)] = -xt, the left side of which is the logit of the earlier quantity. From this equation one can obtain the calculation of t, which is an estimate of the half-life (t1/2) of the effect of the applied agent. In the case of the monotonous curves (sinapic acid, syringic acid, protocatechuic acid, and 3,4-dihydroxy-phenylacetic acid), this t1/2 was varying between 0.9 and 1.3 days. In the case of ferulic acid, in which the observed curve follows that of sinapic acid and ferulic acid for short incubation times and jumps to that of protocatechuic acid and 3,4-dihydroxy-phenylacetic acid for longer incubation times, the calculated t1/2 was 5.7 days. Finally, for caffeic acid, in which a sigmoidal curve was observed, data were not well fitted to the curve and the calculated t1/2 was 4.1 days. (b) Dose effect of phenolic acids on T47D cell proliferation. Cells (2 × 104) were incubated for 5 days in the absence or the presence of the indicated concentrations of phenolic acids, ranging from 10-12 to 10-6 M. The figure presents the ratio of the number of cells in the presence/absence of the indicated phenolic acids. A typical experiment is presented, repeated three more times with similar results.
, in which y is the observed ratio of the cell number in the presence/absence of the phenolic acid, y0 is the maximal observed decrease of cell proliferation (plateau), A1 is 1 - y0, x0 is the initial time of observation (= 0), and x is the time (in days) of the y observation (abscissa of the y observation). The equation after transformation is rearranged as ln [(y - y0) / (1 - y0)] = -xt, the left side of which is the logit of the earlier quantity. From this equation one can obtain the calculation of t, which is an estimate of the half-life (t1/2) of the effect of the applied agent. In the case of the monotonous curves (sinapic acid, syringic acid, protocatechuic acid, and 3,4-dihydroxy-phenylacetic acid), this t1/2 was varying between 0.9 and 1.3 days. In the case of ferulic acid, in which the observed curve follows that of sinapic acid and ferulic acid for short incubation times and jumps to that of protocatechuic acid and 3,4-dihydroxy-phenylacetic acid for longer incubation times, the calculated t1/2 was 5.7 days. Finally, for caffeic acid, in which a sigmoidal curve was observed, data were not well fitted to the curve and the calculated t1/2 was 4.1 days. (b) Dose effect of phenolic acids on T47D cell proliferation. Cells (2 × 104) were incubated for 5 days in the absence or the presence of the indicated concentrations of phenolic acids, ranging from 10-12 to 10-6 M. The figure presents the ratio of the number of cells in the presence/absence of the indicated phenolic acids. A typical experiment is presented, repeated three more times with similar results.
References
-
- Kampa M, Nistikaki A, Hatzoglou A, Kouimtzoglou H, Boskou D, Vercauteren J, Gravanis A, Castanas E. Phenolic acids and polyphenols are potent inhibitors of cancer cell proliferation: assessment and mechanisms of action. In: El-Hadrami I, Daayf F, editor. In Polyphenols 2002: Recent Advances in Polyphenols Research. Marrakech, Morocco: Groupe Polyphenols; 2002. pp. 62–93.
-
- Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. - PubMed
-
- Reiners JJ, Jr, Lee JY, Clift RE, Dudley DT, Myrand SP. PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol Pharmacol. 1998;53:438–445. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

