Malignant myoepithelial cells are associated with the differentiated papillary structure and metastatic ability of a syngeneic murine mammary adenocarcinoma model
- PMID: 14979922
- PMCID: PMC400656
- DOI: 10.1186/bcr757
Malignant myoepithelial cells are associated with the differentiated papillary structure and metastatic ability of a syngeneic murine mammary adenocarcinoma model
Abstract
Background: The normal duct and lobular system of the mammary gland is lined with luminal and myoepithelial cell types. Although evidence suggests that myoepithelial cells might suppress tumor growth, invasion and angiogenesis, their role remains a major enigma in breast cancer biology and few models are currently available for exploring their influence. Several years ago a spontaneous transplantable mammary adenocarcinoma (M38) arose in our BALB/c colony; it contains a malignant myoepithelial cell component and is able to metastasize to draining lymph nodes and lung.
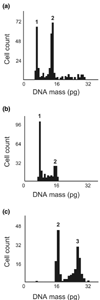
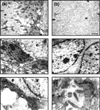
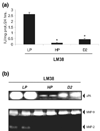
Methods: To characterize this tumor further, primary M38 cultures were established. The low-passage LM38-LP subline contained two main cell components up to the 30th subculture, whereas the higher passage LM38-HP subline was mainly composed of small spindle-shaped cells. In addition, a large spindle cell clone (LM38-D2) was established by dilutional cloning of the low-passage MM38-LP cells. These cell lines were studied by immunocytochemistry, electron microscopy and ploidy, and syngeneic mice were inoculated subcutaneously and intravenously with the different cell lines, either singly or combined to establish their tumorigenic and metastatic capacity.
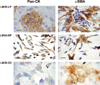
Results: The two subpopulations of LM38-LP cultures were characterized as luminal and myoepithelium-like cells, whereas LM38-HP was mainly composed of small, spindle-shaped epithelial cells and LM38-D2 contained only large myoepithelial cells. All of them were tumorigenic when inoculated into syngeneic mice, but only LM38-LP cultures containing both conserved luminal and myoepithelial malignant cells developed aggressive papillary adenocarcinomas that spread to lung and regional lymph nodes.
Conclusion: The differentiated histopathology and metastatic ability of the spontaneous transplantable M38 murine mammary tumor is associated with the presence and/or interaction of both luminal and myoepithelial tumor cell types.
Figures






References
-
- Liu QY, Niranjan B, Gomes P, Gomm J, Davies D, Coombes C, Buluwela L. Inhibitory effects of activin on the growth and morphogenesis of primary and transformed mammary epithelial cells. Cancer Res. 1996;56:1155–1163. - PubMed
-
- Xiao G, Liu YE, Gentz R, sang QA, Ni J, Goldberg ID, Shi YE. Suppression of breast cancer growth and metastasis by a serpin myoepithelium derived serine protease inhibitor expressed in the mammary myoepithelial cells. Proc Natl Acad Sci. 1999;96:3700–3705. doi: 10.1073/pnas.96.7.3700. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

