Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells
- PMID: 14981092
- PMCID: PMC2172165
- DOI: 10.1083/jcb.200312028
Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells
Abstract
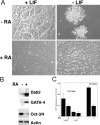
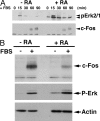
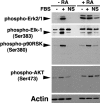
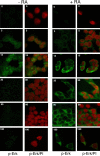
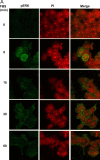
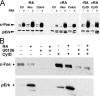
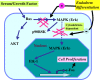
In response to retinoic acid, embryonic stem and carcinoma cells undergo differentiation to embryonic primitive endoderm cells, accompanied by a reduction in cell proliferation. Differentiation does not reduce the activation of cellular MAPK/Erk, but does uncouple mitogen-activated protein kinase (MAPK) activation from phosphorylation/activation of Elk-1 and results in inhibition of c-Fos expression, whereas phosphorylation of the cytoplasmic substrate p90RSK remains unaltered. Cell fractionation and confocal immunofluorescence microscopy demonstrated that activated MAPK is restricted to the cytoplasmic compartment after differentiation. An intact actin and microtubule cytoskeleton appears to be required for the restriction of MAPK nuclear entry induced by retinoic acid treatment because the cytoskeletal disrupting agents nocodazole, colchicine, and cytochalasin D are able to revert the suppression of c-Fos expression. Thus, suppression of cell proliferation after retinoic acid-induced endoderm differentiation of embryonic stem and carcinoma cells is achieved by restricting nuclear entry of activated MAPK, and an intact cytoskeleton is required for the restraint.
Figures





Similar articles
-
Disassociation of MAPK activation and c-Fos expression in F9 embryonic carcinoma cells following retinoic acid-induced endoderm differentiation.J Biol Chem. 2001 Aug 24;276(34):32094-100. doi: 10.1074/jbc.M105009200. Epub 2001 Jun 11. J Biol Chem. 2001. PMID: 11402055
-
Ras/MAPK pathway confers basement membrane dependence upon endoderm differentiation of embryonic carcinoma cells.J Biol Chem. 2002 Oct 25;277(43):40911-8. doi: 10.1074/jbc.M205178200. Epub 2002 Jul 26. J Biol Chem. 2002. PMID: 12145292
-
Compartment-specific regulation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinases (MAPKs) by ERK-dependent and non-ERK-dependent inductions of MAPK phosphatase (MKP)-3 and MKP-1 in differentiating P19 cells.Biochem J. 2000 Dec 15;352 Pt 3(Pt 3):701-8. Biochem J. 2000. PMID: 11104676 Free PMC article.
-
Establishment of ionic channels and signalling cascades in the embryonic stem cell-derived primitive endoderm and cardiovascular system.Cells Tissues Organs. 1999;165(3-4):153-64. doi: 10.1159/000016695. Cells Tissues Organs. 1999. PMID: 10592387 Review.
-
[Microautophagy in the visceral endoderm regurates nutritional provision and differentiation signals during mouse early development].Seikagaku. 2014 Dec;86(6):778-82. Seikagaku. 2014. PMID: 25675817 Review. Japanese. No abstract available.
Cited by
-
Multi-Compartmentalisation in the MAPK Signalling Pathway Contributes to the Emergence of Oscillatory Behaviour and to Ultrasensitivity.PLoS One. 2016 May 31;11(5):e0156139. doi: 10.1371/journal.pone.0156139. eCollection 2016. PLoS One. 2016. PMID: 27243235 Free PMC article.
-
Retinoic acid inhibits airway smooth muscle cell migration.Am J Respir Cell Mol Biol. 2006 Jun;34(6):695-703. doi: 10.1165/rcmb.2005-0306OC. Epub 2006 Feb 2. Am J Respir Cell Mol Biol. 2006. PMID: 16456186 Free PMC article.
-
Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors.J Biol Chem. 2006 Nov 3;281(44):33749-60. doi: 10.1074/jbc.M603862200. Epub 2006 Sep 1. J Biol Chem. 2006. PMID: 16954126 Free PMC article.
-
Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons.PLoS One. 2012;7(1):e29239. doi: 10.1371/journal.pone.0029239. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235275 Free PMC article.
-
Retinoic Acid Induces Differentiation of Mouse F9 Embryonic Carcinoma Cell by Modulating the miR-485 Targeting of Abhd2.Int J Mol Sci. 2019 Apr 26;20(9):2071. doi: 10.3390/ijms20092071. Int J Mol Sci. 2019. PMID: 31035455 Free PMC article.
References
-
- Angel, P., and M. Karin. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1072:129–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

