Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir
- PMID: 14982766
- PMCID: PMC353103
- DOI: 10.1128/AAC.48.3.791-798.2004
Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir
Abstract
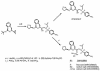
GW433908 is the water-soluble, phosphate ester prodrug of the human immunodeficiency virus type 1 protease inhibitor amprenavir (APV). A high-yield synthesis of GW433908 is achieved by phosphorylation of the penultimate precursor of APV with phosphorous oxychloride (POCl(3)) in pyridine. A single-dose pharmacokinetic study of GW433908 sodium salt in dogs showed that APV exposure was similar to that achieved with an equivalent molar dose of the APV clinical formulation (Agenerase) and that systemic exposure to the prodrug was minimal (0.3% of the APV exposure). However, the sodium salt of GW433908 was a hygroscopic, amorphous solid and thus not suitable for pharmaceutical development. The calcium salt was a developable crystalline solid, but oral dosing afforded only 24% of the APV exposure in dogs compared with Agenerase. Acidification of the dog stomach by coadministration of HCl increased the bioavailability of the calcium salt to levels near those of the sodium salt. Single-dose administration of GW433908 calcium salt in dogs and rats produced portal vein GW433908 concentrations that were maximally 1.72 and 0.79% of those of APV concentrations, respectively. Furthermore, GW433908 had poor transepithelial flux and APV showed significant flux across human-derived Caco-2 cell monolayers (a model of intestinal permeability). Taken together, these results suggest that GW433908 is primarily metabolized to APV at or in the epithelial cells of the intestine and that the prodrug is not substantially absorbed. Based in part on these findings, GW433908 was advanced to clinical development.
Figures
References
-
- Bangsberg, D. R., F. M. Hecht, E. D. Charlebois, A. R. Zolopa, M. Holodniy, L. Sheiner, J. D. Bamberger, M. A. Chesney, and A. Moss. 2000. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 14:357-366. - PubMed
-
- Bartlett, J. A., R. DeMasi, J. Quinn, C. Moxham, and F. Rousseau. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naïve HIV-1 infected adults. AIDS 15:1369-1377. - PubMed
-
- Brodt, H. R., B. S. Kamps, P. Gute, B. Knupp, S. Staszewski, and E. B. Helm. 1997. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS 11:1731-1738. - PubMed
-
- Centers for Disease Control and Prevention. 1997. Update: trends in AIDS incidence—United States, 1996. Morb. Mortal. Wkly. Rep. 46:861-864. - PubMed
-
- Claxton, A. J., J. Cramer, and C. Pierce. 2001. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 23:1296-1310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources