Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men
- PMID: 14982768
- PMCID: PMC353067
- DOI: 10.1128/AAC.48.3.804-808.2004
Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men
Abstract
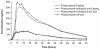
Posaconazole is a potent broad-spectrum azole antifungal agent in clinical development for the treatment of invasive fungal infections. This study evaluated the potential for a pH-dependent pharmacokinetic interaction between posaconazole and an antacid (Mylanta), under fasting and nonfasting conditions. Twelve men completed this randomized, four-period crossover, single-dose study. Subjects received 200 mg of posaconazole following a 10-h fast, with 20 ml of Mylanta and a 10-h fast, with 20 ml of Mylanta and a high-fat breakfast, and with a high-fat breakfast alone. Antacid coadministration had no statistically significant effects on posaconazole bioavailability under fasting or nonfasting conditions. In the fasting state, antacid slightly increased the relative oral bioavailability of posaconazole by 15% (P = 0.296); in the nonfasting state, antacid decreased the relative bioavailability of posaconazole by 12% (P = 0.352). Food increased the relative oral bioavailability of posaconazole by 400% (P = 0.001). In conclusion, the effect of antacid on posaconazole exposure in the fasting or nonfasting state was small and is not considered clinically significant.
Figures

References
-
- Barchiesi, F., A. M. Schimizzi, F. Caselli, D. Giannini, V. Camiletti, B. Fileni, A. Giacometti, L. F. Di Francesco, and G. Scalise. 2001. Activity of the new antifungal triazole, posaconazole, against Cryptococcus neoformans. J. Antimicrob. Chemother. 48:769-773. - PubMed
-
- Barone, J. A., J. G. Koh, R. H. Bierman, J. L. Colaizzi, K. A. Swanson, M. C. Gaffar, B. L. Moskovitz, W. Mechlinski, and V. Van de Velde. 1993. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob. Agents Chemother. 37:778-784. - PMC - PubMed
-
- Barone, J. A., B. L. Moskovitz, J. Guarnieri, A. E. Hassell, J. L. Colaizzi, R. H. Bierman, and L. Jessen. 1998. Food interaction and steady-state pharmacokinetics of itraconazole oral solution in healthy volunteers. Pharmacotherapy 18:295-301. - PubMed
-
- Blum, R. A., D. T. D'Andrea, B. M. Florentino, J. H. Wilton, D. M. Hilligoss, M. J. Gardner, E. B. Henry, H. Goldstein, and J. J. Schentag. 1991. Increased gastric pH and the bioavailability of fluconazole and ketoconazole. Ann. Intern. Med. 114:755-757. - PubMed
-
- Carlson, J. A., H. J. Mann, and D. M. Canafax. 1983. Effect of pH on disintegration and dissolution of ketoconazole tablets. Am. J. Hosp. Pharm. 40:1334-1336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

