Human pancreatic islet-derived progenitor cell engraftment in immunocompetent mice
- PMID: 14982836
- PMCID: PMC1613272
- DOI: 10.1016/S0002-9440(10)63170-7
Human pancreatic islet-derived progenitor cell engraftment in immunocompetent mice
Abstract
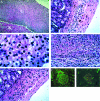
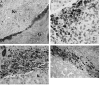
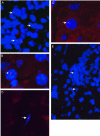
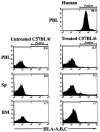
The potential for the use of stem/progenitor cells for the restoration of injured or diseased tissues has garnered much interest recently, establishing a new field of research called regenerative medicine. Attention has been focused on embryonic stem cells derived from human fetal tissues. However, the use of human fetal tissue for research and transplantation is controversial. An alternative is the isolation and utilization of multipotent stem/progenitor cells derived from adult donor tissues. We have previously reported on the isolation, propagation, and partial characterization of a population of stem/progenitor cells isolated from the pancreatic islets of Langerhans of adult human donor pancreata. Here we show that these human adult tissue-derived cells, nestin-positive islet-derived stem/progenitor cells, prepared from human adult pancreata survive engraftment and produce tissue chimerism when transplanted into immunocompetent mice either under the kidney capsule or by systemic injection. These xenografts seem to induce immune tolerance by establishing a mixed chimerism in the mice. We propose that a population of stem/progenitor cells isolated from the islets of the pancreas can cross xenogeneic transplantation immune barriers, induce tissue tolerance, and grow.
Figures





Similar articles
-
Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes.Diabetes. 2001 Mar;50(3):521-33. doi: 10.2337/diabetes.50.3.521. Diabetes. 2001. PMID: 11246871
-
Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter.Biochem Biophys Res Commun. 2002 May 3;293(2):670-4. doi: 10.1016/S0006-291X(02)00275-9. Biochem Biophys Res Commun. 2002. PMID: 12054520
-
Islet neogenesis from the constitutively nestin expressing human umbilical cord matrix derived mesenchymal stem cells.Islets. 2010 Mar-Apr;2(2):112-20. doi: 10.4161/isl.2.2.11280. Islets. 2010. PMID: 21099303
-
Stem cells with potential to generate insulin producing cells in man.Swiss Med Wkly. 2006 Oct 14;136(41-42):647-54. doi: 10.4414/smw.2006.11425. Swiss Med Wkly. 2006. PMID: 17103343 Review.
-
Stem cell-based approaches to solving the problem of tissue supply for islet transplantation in type 1 diabetes.Int J Biochem Cell Biol. 2004 Apr;36(4):667-83. doi: 10.1016/j.biocel.2003.09.005. Int J Biochem Cell Biol. 2004. PMID: 15010331 Review.
Cited by
-
Immune-privileged embryonic Swiss mouse STO and STO cell-derived progenitor cells: major histocompatibility complex and cell differentiation antigen expression patterns resemble those of human embryonic stem cell lines.Immunology. 2006 Sep;119(1):98-115. doi: 10.1111/j.1365-2567.2006.02412.x. Epub 2006 Jul 10. Immunology. 2006. PMID: 16836618 Free PMC article.
-
Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques.Organogenesis. 2011 Jul-Sep;7(3):154-62. doi: 10.4161/org.7.3.16522. Epub 2011 Jul 1. Organogenesis. 2011. PMID: 21654197 Free PMC article.
-
Xenotransplantation of pancreatic and kidney primordia-where do we stand?Transpl Immunol. 2009 Jun;21(2):93-100. doi: 10.1016/j.trim.2008.10.007. Epub 2008 Nov 6. Transpl Immunol. 2009. PMID: 18992818 Free PMC article.
-
Human islet-derived precursor cells can cycle between epithelial clusters and mesenchymal phenotypes.J Cell Mol Med. 2009 Aug;13(8B):2570-2581. doi: 10.1111/j.1582-4934.2008.00570.x. Epub 2008 Nov 3. J Cell Mol Med. 2009. PMID: 19175683 Free PMC article.
-
Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas.Am J Physiol Gastrointest Liver Physiol. 2010 Aug;299(2):G303-10. doi: 10.1152/ajpgi.00146.2010. Epub 2010 Jun 3. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20522640 Free PMC article.
References
-
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
-
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. - PubMed
-
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. - PubMed
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Slack JM. Stem cells in epithelial tissues. Science. 2000;287:1431–1433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

