Secondary coronary artery vasospasm promotes cardiomyopathy progression
- PMID: 14982859
- PMCID: PMC1614719
- DOI: 10.1016/S0002-9440(10)63193-8
Secondary coronary artery vasospasm promotes cardiomyopathy progression
Abstract
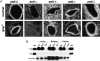
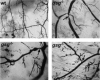
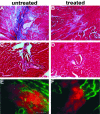
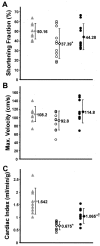
Genetic defects in the plasma membrane-associated sarcoglycan complex produce cardiomyopathy characterized by focal degeneration. The infarct-like pattern of cardiac degeneration has led to the hypothesis that coronary artery vasospasm underlies cardiomyopathy in this disorder. We evaluated the coronary vasculature of gamma-sarcoglycan mutant mice and found microvascular filling defects consistent with arterial vasospasm. However, the vascular smooth muscle sarcoglycan complex was intact in the coronary arteries of gamma-sarcoglycan hearts with perturbation of the sarcoglycan complex only within the adjacent myocytes. Thus, in this model, coronary artery vasospasm derives from a vascular smooth muscle-cell extrinsic process. To reduce this secondary vasospasm, we treated gamma-sarcoglycan-deficient mice with the calcium channel antagonist verapamil. Verapamil treatment eliminated evidence of vasospasm and ameliorated histological and functional evidence of cardiomyopathic progression. Echocardiography of verapamil-treated, gamma-sarcoglycan-null mice showed an improvement in left ventricular fractional shortening (44.3 +/- 13.3% treated versus 37.4 +/- 15.3% untreated), maximal velocity at the aortic outflow tract (114.9 +/- 27.9 cm/second versus 92.8 +/- 22.7 cm/second), and cardiac index (1.06 +/- 0.30 ml/minute/g versus 0.67 +/- 0.16 ml/minute/g, P < 0.05). These data indicate that secondary vasospasm contributes to the development of cardiomyopathy and is an important therapeutic target to limit cardiomyopathy progression.
Figures

Similar articles
-
Smooth muscle cell-extrinsic vascular spasm arises from cardiomyocyte degeneration in sarcoglycan-deficient cardiomyopathy.J Clin Invest. 2004 Mar;113(5):668-75. doi: 10.1172/JCI20410. J Clin Invest. 2004. PMID: 14991064 Free PMC article.
-
Prevention of cardiomyopathy in mouse models lacking the smooth muscle sarcoglycan-sarcospan complex.J Clin Invest. 2001 Jan;107(2):R1-7. doi: 10.1172/JCI11642. J Clin Invest. 2001. PMID: 11160141 Free PMC article.
-
Cardiomyopathy is independent of skeletal muscle disease in muscular dystrophy.FASEB J. 2002 Jul;16(9):1096-8. doi: 10.1096/fj.01-0954fje. Epub 2002 May 8. FASEB J. 2002. PMID: 12039854
-
Sarcoglycans in muscular dystrophy.Microsc Res Tech. 2000 Feb 1-15;48(3-4):167-80. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<167::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10679964 Review.
-
Sarcoglycans in vascular smooth and striated muscle.Trends Cardiovasc Med. 2003 Aug;13(6):238-43. doi: 10.1016/s1050-1738(03)00101-4. Trends Cardiovasc Med. 2003. PMID: 12922020 Review.
Cited by
-
Cardiac function in muscular dystrophy associates with abdominal muscle pathology.J Neuromuscul Dis. 2015;2(1):39-49. doi: 10.3233/JND-140062. J Neuromuscul Dis. 2015. PMID: 26029630 Free PMC article.
-
Drosophila as a model for the identification of genes causing adult human heart disease.Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1394-9. doi: 10.1073/pnas.0507359103. Epub 2006 Jan 23. Proc Natl Acad Sci U S A. 2006. PMID: 16432241 Free PMC article.
-
Hemodynamic alterations in the coronary circulation of cardiomyopathic hamsters: age and Ang II-dependent mechanisms.J Card Fail. 2009 Dec;15(10):929-38. doi: 10.1016/j.cardfail.2009.06.441. Epub 2009 Aug 20. J Card Fail. 2009. PMID: 19944371 Free PMC article.
-
Coronary microthrombi in the failing human heart: the role of von Willebrand factor and PECAM-1.Mol Cell Biochem. 2024 Dec;479(12):3437-3446. doi: 10.1007/s11010-024-04942-0. Epub 2024 Feb 21. Mol Cell Biochem. 2024. PMID: 38381272 Free PMC article.
-
Syntrophins entangled in cytoskeletal meshwork: Helping to hold it all together.Cell Prolif. 2019 Mar;52(2):e12562. doi: 10.1111/cpr.12562. Epub 2018 Dec 4. Cell Prolif. 2019. PMID: 30515904 Free PMC article. Review.
References
-
- Heydemann A, Wheeler MT, McNally EM. Cardiomyopathy in animal models of muscular dystrophy. Curr Opin Cardiol. 2001;16:211–217. - PubMed
-
- Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–343. - PubMed
-
- Rafael JA, Brown SC. Dystrophin and utrophin: genetic analyses of their role in skeletal muscle. Microsc Res Tech. 2000;48:155–166. - PubMed
-
- Bonnemann CG, McNally EM, Kunkel LM. Beyond dystrophin: current progress in the muscular dystrophies. Curr Opin Pediatr. 1996;8:569–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

