Transcriptional regulation of the Drosophila catalase gene by the DRE/DREF system
- PMID: 14982956
- PMCID: PMC390290
- DOI: 10.1093/nar/gkh302
Transcriptional regulation of the Drosophila catalase gene by the DRE/DREF system
Abstract
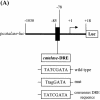
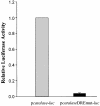
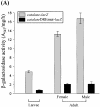
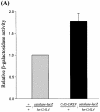
Reactive oxygen species (ROS) cause oxidative stress and aging. The catalase gene is a key component of the cellular antioxidant defense network. However, the molecular mechanisms that regulate catalase gene expression are poorly understood. In this study, we have identified a DNA replication-related element (DRE; 5'-TATCGATA) in the 5'-flanking region of the Drosophila catalase gene. Gel mobility shift assays revealed that a previously identified factor called DREF (DRE- binding factor) binds to the DRE sequence in the Drosophila catalase gene. We used site-directed mutagenesis and in vitro transient transfection assays to establish that expression of the catalase gene is regulated by DREF through the DRE site. To explore the role of DRE/DREF in vivo, we established transgenic flies carrying a catalase-lacZ fusion gene with or without mutation in the DRE. The beta-galactosidase expression patterns of these reporter transgenic lines demonstrated that the catalase gene is upregulated by DREF through the DRE sequence. In addition, we observed suppression of the ectopic DREF-induced rough eye phenotype by a catalase amorphic Cat(n1) allele, indicating that DREF activity is modulated by the intracellular redox state. These results indicate that the DRE/DREF system is a key regulator of catalase gene expression and provide evidence of cross-talk between the DRE/DREF system and the antioxidant defense system.
Figures




Similar articles
-
Transcriptional regulation of the Drosophila ANT gene by the DRE/DREF system.Genes Cells. 2007 May;12(5):569-79. doi: 10.1111/j.1365-2443.2007.01075.x. Genes Cells. 2007. PMID: 17535248
-
Role of DREF in transcriptional regulation of the Drosophila p53 gene.Oncogene. 2010 Apr 8;29(14):2060-9. doi: 10.1038/onc.2009.483. Epub 2010 Jan 18. Oncogene. 2010. PMID: 20101238
-
Identification of the Drosophila eIF4A gene as a target of the DREF transcription factor.Exp Cell Res. 2007 Dec 10;313(20):4208-20. doi: 10.1016/j.yexcr.2007.08.016. Epub 2007 Aug 24. Exp Cell Res. 2007. PMID: 17888422
-
The DRE/DREF transcriptional regulatory system: a master key for cell proliferation.Biochim Biophys Acta. 2008 Feb;1779(2):81-9. doi: 10.1016/j.bbagrm.2007.11.011. Epub 2007 Dec 4. Biochim Biophys Acta. 2008. PMID: 18155677 Review.
-
ZBED1/DREF: A transcription factor that regulates cell proliferation.Oncol Lett. 2020 Nov;20(5):137. doi: 10.3892/ol.2020.11997. Epub 2020 Aug 20. Oncol Lett. 2020. PMID: 32934705 Free PMC article. Review.
Cited by
-
DREF is involved in the steroidogenesis via regulation of shadow gene.Am J Cancer Res. 2012;2(6):714-25. Epub 2012 Nov 20. Am J Cancer Res. 2012. PMID: 23226617 Free PMC article.
-
Excitation-transcription coupling, neuronal gene expression and synaptic plasticity.Nat Rev Neurosci. 2023 Nov;24(11):672-692. doi: 10.1038/s41583-023-00742-5. Epub 2023 Sep 29. Nat Rev Neurosci. 2023. PMID: 37773070 Free PMC article. Review.
-
Modulation of fracture healing by senescence-associated secretory phenotype (SASP): a narrative review of the current literature.Eur J Med Res. 2024 Jan 9;29(1):38. doi: 10.1186/s40001-023-01604-7. Eur J Med Res. 2024. PMID: 38195489 Free PMC article. Review.
-
Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes.Genome Biol. 2007;8(12):R262. doi: 10.1186/gb-2007-8-12-r262. Genome Biol. 2007. PMID: 18067683 Free PMC article.
-
Phosphine inhibits transcription of the catalase gene through the DRE/DREF system in Drosophila melanogaster.Sci Rep. 2017 Oct 10;7(1):12913. doi: 10.1038/s41598-017-13439-4. Sci Rep. 2017. PMID: 29018235 Free PMC article.
References
-
- Mates J.M., Perez-Gomez,C. and Nunez de Castro,I. (1999) Antioxidant enzymes and human diseases. Clin. Biochem., 32, 595–603. - PubMed
-
- Monnier V., Girardot,F., Audin,W. and Tricoire,H. (2002) Control of oxidative stress resistance by IP3 kinase in Drosophila melanogaster. Free Radic. Biol. Med., 33, 1250–1259. - PubMed
-
- Michiels C., Raes,M., Toussaint,O. and Remacle,J. (1994) Importance of Se-glutathione peroxidase, catalase and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med., 17, 235–248. - PubMed
-
- Aebi H. (1984) Catalase in vitro. Methods Enzymol., 105, 121–126. - PubMed
-
- Orr W.C. and Sohal,R.S. (1994) Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science, 263, 1128–1130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

