Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo
- PMID: 14982997
- PMCID: PMC356938
- DOI: 10.1073/pnas.0308762101
Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo
Abstract
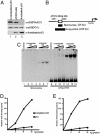
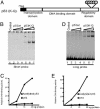
p53 promotes tumor suppression through its ability to function as a transcriptional factor and is activated by posttranslational modifications that include acetylation. Our earlier study demonstrated that p53 acetylation can enhance its sequence-specific DNA binding in vitro, and this notion was later confirmed in several other studies. However, a recent study has reported that in vitro acetylation of p53 fails to stimulate its DNA binding to large DNA fragments, raising an important issue that requires further investigation. Here, we show that unacetylated p53 is able to bind weakly to its consensus site within the context of large DNA fragments, although it completely fails to bind the same site within short oligonucleotide probes. Strikingly, by using highly purified and fully acetylated p53 proteins obtained from cells, we show that acetylation of the C-terminal domain can dramatically enhance site-specific DNA binding on both short oligonucleotide probes and long DNA fragments. Moreover, endogenous p53 apparently can be fully acetylated in response to DNA damage when both histone deacetylase complex 1 (HDAC1)- and Sir2-mediated deacetylation are inhibited, indicating dynamic p53 acetylation and deacetylation events during the DNA damage response. Finally, we also show that acetylation of endogenous p53 indeed significantly augments its ability to bind an endogenous target gene and that p53 acetylation levels correlate well with p53-mediated transcriptional activation in vivo. Thus, our results clarify some of the confusion surrounding acetylation-mediated effects on p53 binding to DNA and suggest that acetylation of p53 in vivo may contribute, at least in part, to its transcriptional activation functions.
Figures
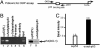
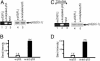

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

