Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo
- PMID: 14983007
- PMCID: PMC356948
- DOI: 10.1073/pnas.0308463100
Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo
Abstract
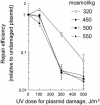
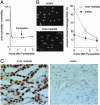
Acute exposure of cells in culture to high NaCl damages DNA and impairs its repair. However, after several hours of cell cycle arrest, cells multiply in the hypertonic medium. Here, we show that, although adapted cells proliferate rapidly and do not become apoptotic, they nevertheless contain numerous DNA breaks, which do not elicit a DNA damage response. Thus, in adapted cells, Mre11 exonuclease is mainly present in the cytoplasm, rather than nucleus, and histone H2AX and chk1 are not phosphorylated, as they normally would be in response to DNA damage. Also, the adapted cells are deficient in repair of luciferase reporter plasmids damaged by UV irradiation. On the other hand, the DNA damage response activates rapidly when the level of NaCl is reduced. Then, Mre11 moves into the nucleus, and H2AX and chk1 become phosphorylated. Renal inner medullary cells in vivo are normally exposed to a variable, but always high, level of NaCl. As with adapted cells in culture, inner medullary cells in normal mice exhibit numerous DNA breaks. These DNA breaks are rapidly repaired when the NaCl level is decreased by injection of the diuretic furosemide. Moreover, repair of DNA breaks induced by ionizing radiation is inhibited in the inner medulla. Histone H2AX does not become phosphorylated, and repair synthesis is not detectable in response to total body irradiation unless NaCl is lowered by furosemide. Thus, both in cell culture and in vivo, although cells adapt to high NaCl, their DNA is damaged and its repair is inhibited.
Figures




Similar articles
-
High NaCl causes Mre11 to leave the nucleus, disrupting DNA damage signaling and repair.Am J Physiol Renal Physiol. 2003 Aug;285(2):F266-74. doi: 10.1152/ajprenal.00060.2003. Epub 2003 Apr 8. Am J Physiol Renal Physiol. 2003. PMID: 12684226
-
Hypertonic stress response.Mutat Res. 2005 Jan 6;569(1-2):65-74. doi: 10.1016/j.mrfmmm.2004.06.053. Mutat Res. 2005. PMID: 15603752 Review.
-
Living with DNA breaks is an everyday reality for cells adapted to high NaCl.Cell Cycle. 2004 May;3(5):561-3. Epub 2004 May 19. Cell Cycle. 2004. PMID: 15107607
-
Analysis of DNA breaks, DNA damage response, and apoptosis produced by high NaCl.Am J Physiol Renal Physiol. 2008 Dec;295(6):F1678-88. doi: 10.1152/ajprenal.90424.2008. Epub 2008 Oct 1. Am J Physiol Renal Physiol. 2008. PMID: 18829739 Free PMC article.
-
DNA damage and osmotic regulation in the kidney.Am J Physiol Renal Physiol. 2005 Jul;289(1):F2-7. doi: 10.1152/ajprenal.00041.2005. Am J Physiol Renal Physiol. 2005. PMID: 15951478 Review.
Cited by
-
NFAT5 in cellular adaptation to hypertonic stress - regulations and functional significance.J Mol Signal. 2013 Apr 23;8(1):5. doi: 10.1186/1750-2187-8-5. J Mol Signal. 2013. PMID: 23618372 Free PMC article.
-
How do kinases contribute to tonicity-dependent regulation of the transcription factor NFAT5?World J Nephrol. 2016 Jan 6;5(1):20-32. doi: 10.5527/wjn.v5.i1.20. World J Nephrol. 2016. PMID: 26788461 Free PMC article. Review.
-
Somatic mutation in autosomal dominant polycystic kidney disease revealed by deep sequencing human kidney cysts.NPJ Genom Med. 2024 Dec 19;9(1):69. doi: 10.1038/s41525-024-00452-6. NPJ Genom Med. 2024. PMID: 39702469 Free PMC article.
-
Angiotensin II Exposure In Vitro Reduces High Salt-Induced Reactive Oxygen Species Production and Modulates Cell Adhesion Molecules' Expression in Human Aortic Endothelial Cell Line.Biomedicines. 2024 Nov 29;12(12):2741. doi: 10.3390/biomedicines12122741. Biomedicines. 2024. PMID: 39767646 Free PMC article.
-
Potential Role of Gene Regulator NFAT5 in the Pathogenesis of Diabetes Mellitus.J Diabetes Res. 2020 Sep 15;2020:6927429. doi: 10.1155/2020/6927429. eCollection 2020. J Diabetes Res. 2020. PMID: 33015193 Free PMC article. Review.
References
-
- Zhu, J., Petersen, S., Tessarollo, L. & Nussenzweig, A. (2001) Curr. Biol. 11, 105–109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

