The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis
- PMID: 14983012
- PMCID: PMC356953
- DOI: 10.1073/pnas.0308065101
The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis
Abstract
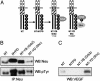
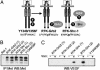
The etiology and progression of a variety of human malignancies are linked to the deregulation of receptor tyrosine kinases (RTKs). To define the role of RTK-dependent signals in various oncogenic processes, we have previously engineered RTK oncoproteins that recruit either the Shc or Grb2 adaptor proteins. Although these RTK oncoproteins transform cells with similar efficiencies, fibroblasts expressing the Shc-binding RTK oncoproteins induced tumors with short latency (approximately 7 days), whereas cells expressing the Grb2-binding RTK oncoproteins induced tumors with delayed latency (approximately 24 days). The early onset of tumor formation correlated with the ability of cells expressing the Shc-binding RTK oncoproteins to produce vascular endothelial growth factor (VEGF) in culture and an angiogenic response in vivo. Consistent with this, treatment with a VEGF inhibitor, VEGF-Trap, blocked the in vivo angiogenic and tumorigenic properties of these cells. The importance of Shc recruitment to RTKs for the induction of VEGF was further demonstrated by using mutants of the Neu/ErbB2 RTK, where the Shc, but not Grb2, binding mutant induced VEGF. Moreover, the use of fibroblasts derived from ShcA-deficient mouse embryos, demonstrated that Shc was essential for the induction of VEGF by the Met/hepatocyte growth factor RTK oncoprotein and by serum-derived growth factors. Together, our findings identify Shc as a critical angiogenic switch for VEGF production downstream from the Met and ErbB2 RTKs.
Figures




References
-
- Blume-Jensen, P. & Hunter, T. (2001) Nature 411, 355–365. - PubMed
-
- Pawson, T. & Nash, P. (2000) Genes Dev. 14, 1027–1047. - PubMed
-
- Saucier, C., Papavasiliou, V., Palazzo, A., Naujokas, M. A., Kremer, R. & Park, M. (2002) Oncogene 21, 1800–1811. - PubMed
-
- Fixman, E. D., Fournier, T. M., Kamikura, D. M., Naujokas, M. A. & Park, M. (1996) J. Biol. Chem. 271, 13116–13122. - PubMed
-
- Pelicci, G., Lanfrancone, L., Grignani, F., McGlade, J., Cavallo, F., Forni, G., Nicoletti, L., Pawson, T. & Pelicci, P. G. (1992) Cell 70, 93–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

