Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association
- PMID: 14983044
- PMCID: PMC356985
- DOI: 10.1073/pnas.0307140101
Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association
Abstract
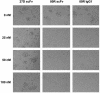
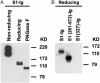
Effective prophylaxis and antiviral therapies are urgently needed in the event of reemergence of the highly contagious and often fatal severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) infection. We have identified eight recombinant human single-chain variable region fragments (scFvs) against the S1 domain of spike (S) protein of the SARS-CoV from two nonimmune human antibody libraries. One scFv 80R efficiently neutralized SARS-CoV and inhibited syncytia formation between cells expressing the S protein and those expressing the SARS-CoV receptor angiotensin-converting enzyme 2 (ACE2). Mapping of the 80R epitope showed it is located within the N-terminal 261-672 amino acids of S protein and is not glycosylation-dependent. 80R scFv competed with soluble ACE2 for association with the S1 domain and bound S1 with high affinity (equilibrium dissociation constant, Kd=32.3 nM). A human IgG1 form of 80R bound S1 with a 20-fold higher affinity of 1.59 nM comparable to that of ACE2 (Kd=1.70 nM), and neutralized virus 20-fold more efficiently than the 80R scFv. These data suggest that the 80R human monoclonal antibody may be a useful viral entry inhibitor for the emergency prophylaxis and treatment of SARS, and that the ACE2-binding site of S1 could be an attractive target for subunit vaccine and drug development.
Figures


References
-
- Ksiazek, T. G., Erdman, D., Goldsmith, C. S., Zaki, S. R., Peret, T., Emery, S., Tong, S., Urbani, C., Comer, J. A., Lim, W., et al. (2003) N. Engl. J. Med. 348, 1953-1966. - PubMed
-
- Drosten, C., Gunther, S., Preiser, W., van der Werf, S., Brodt, H.-R., Becker, S., Rabenau, H., Panning, M., Kolesnikova, L., Fouchier, R. A. M., et al. (2003) N. Engl. J. Med. 348, 1967-1976. - PubMed
-
- Holmes, K. V. (2003) N. Engl. J. Med. 348, 1948-1951. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

