BAG-1--a nucleotide exchange factor of Hsc70 with multiple cellular functions
- PMID: 14984055
- PMCID: PMC514875
- DOI: 10.1379/1466-1268(2003)008<0225:bnefoh>2.0.co;2
BAG-1--a nucleotide exchange factor of Hsc70 with multiple cellular functions
Abstract
BAG-1 (Bcl-2-associated athanogene) is a multifaceted protein implicated in the modulation of a large variety of cellular processes. Elucidating the molecular mechanisms that underlie the cellular functions of BAG-1 becomes an increasingly important task, particularly in light of the growing evidence connecting aberrant BAG-1 expression to certain human cancers. A common element of the remarkable functional diversity of BAG-1 appears to be the interaction with molecular chaperones of the Hsp70 family. In fact, BAG-1 functions as a nucleotide exchange factor of mammalian cytosolic Hsc70, thereby triggering substrate unloading from the chaperone. In addition, recent findings reveal an association of BAG-1 with the proteasome, which suggests a role in coordinating chaperone and degradation pathways.
Figures
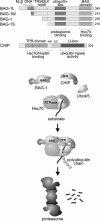
References
-
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, Höhfeld J. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem. 2002;277:45920–45927. - PubMed
-
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. - PubMed
-
- Coldwell MJ, deSchoolmeester ML, Fraser GA, Pickering BM, Packham G, Willis AE. The p36 isoform of BAG-1 is translated by internal ribosome entry following heat shock. Oncogene. 2001;20:4095–4100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
