Induction of heat shock protein gp96 by immune cytokines
- PMID: 14984057
- PMCID: PMC514877
- DOI: 10.1379/1466-1268(2003)008<0242:iohspg>2.0.co;2
Induction of heat shock protein gp96 by immune cytokines
Abstract
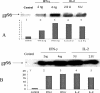
Cytokines play a major role in regulating both humoral and cell-mediated immune responses. Recent advances in our understanding of cell-mediated immune responses have focused on the antigen presentation machinery and the proteins in the endoplasmic reticulum (ER). These proteins help the formation and stabilization of the major histocompatibility complex (MHC)-peptide interaction. A 96-kDa, ER-resident glycoprotein (gp96) is being evaluated as a therapeutic agent in cancer because of its ability to associate with a vast number of cellular peptides irrespective of size or sequence. Because the antigen presentation complex is assembled in the ER and a number of ER-resident proteins are modulated by cytokines, it is important to examine the regulation of gp96 in response to immune cytokines interferon gamma (IFN-gamma), and interleukin 2 (IL-2). Defects in signaling pathway in either of the cytokines can result in suboptimal immune response. We examined the effect of the cytokines IFN-gamma and IL-2 on the induction of gp96 in different cancer cell lines and examined the induction of DNA-binding proteins that recognize gamma interferon-activating sequence (GAS), present in the promoter region of gp96. The induction of GAS binding protein correlated with the induction of STAT 1 protein, a transcriptional regulator and mediator of IFN-gamma-mediated gene expression. The use of cytokines in inducing gp96 levels may have significance in maintaining high levels of gp96 for a sustained immune response.
Figures

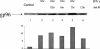


References
-
- Arnold D, Driscoll J, Androlewicz M, Hughes E, Cresswell P, Spies T. Proteasome subunits encoded in the MHC are not generally required for the processing of peptides bound by MHC class I molecules. Nature. 1992;360:171–174. - PubMed
-
- Ashok BT, Chen YG, and Liu X. et al. 2002 Multiple molecular targets of indole-3-carbinol, a chemopreventive anti-estrogen in breast cancer. Eur J Cancer Prev. 11:S86–S93. - PubMed
-
- Ashok BT, Kim E, Mittelman A, Tiwari RK. Proteasome inhibitors differentially affect heat shock protein response in cancer cells. Int J Mol Med. 2001;8:385–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
