Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system
- PMID: 14984061
- PMCID: PMC514881
- DOI: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2
Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system
Abstract
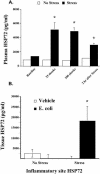
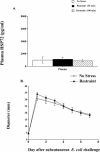
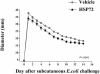
Extracellular heat-shock proteins (eHsp) such as those belonging to the 70-kDa family of Hsp (eg, Hsp72) have been hypothesized to act as a "danger signal" to immune cells, promote immune responses, and improve host defense. The current study tested this hypothesis. Adult male F344 rats were exposed to an acute laboratory stressor (100, 5-second, 1.6-mA inescapable tail shocks) and challenged with Escherichia coli. The number of colony-forming units (CFU) of bacteria at the site of injection, the levels of eHsp72, the immune response to eHsp72 and E. coli-derived lipopolysaccharide (LPS), and the amount of time required to recover from in vivo bacterial challenge were measured. CFUs were reduced 2, 4, and 6 hours after injection of E. coli in rats exposed to stress. Rats exposed to stress had elevated eHsp72 that was elevated rapidly (25 minutes) and remained elevated in the circulation and at the inflammatory site (2 hours after stressor termination). Both stressor exposure and eHsp72 administration in the absence of stress resulted in a facilitated pattern of recovery after bacterial inflammation induced by subcutaneous E. coli injection. Rats exposed to acute restraint (100 minutes) did not demonstrate elevated circulating eHsp72 or a facilitated pattern of recovery after bacterial challenge. In vitro stimulation of rat splenocytes and macrophages with eHsp72 elevated nitric oxide (NO), tumor necrosis factor alpha (TNF-alpha), interleukin (IL)-1beta, and IL-6, and this effect was specific to eHsp72 because it was not diminished by polymyxin B and was reduced by earlier heat-denature treatment. Stimulation of cells with eHsp72 combined with LPS resulted in a greater NO and cytokine response than that observed after stimulation with eHsp72 or LPS alone. In vivo, at the inflammatory site, the bacterial-induced NO response was potentiated by stress, and NO inhibition (L-NIO) reduced the stress-induced facilitation but had no effect on the control kinetics of bacterial inflammation recovery. Thus, these results lend support to the hypothesis that intense stressor exposure increases eHsp72, which acts as a danger signal to potentiate the NO response to bacterial challenge and facilitate recovery from bacterial inflammation.
Figures









References
-
- Ali H, Haribabu B, Richardson RM, Snyderman R. Mechanisms of inflammation and leukocyte activation. Med Clin North Am. 1997;81:1–28. - PubMed
-
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. - PubMed
-
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. - PubMed
-
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
