Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway
- PMID: 14985429
- PMCID: PMC6730405
- DOI: 10.1523/JNEUROSCI.3809-03.2004
Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway
Abstract
Cytoplasmic deposition of alpha-synuclein aggregates is a common pathological feature of many neurodegenerative diseases. Strong evidence for the causative role of alpha-synuclein in these disorders is provided by genetic linkage between this gene and familial Parkinson's disease and by neurodegeneration in transgenic animals that overexpress this protein. In particular, it has been hypothesized that the accumulation of nonfibrillar oligomers of alpha-synuclein, which serve as intermediates for fibrillar inclusion body formation, causes neurodegeneration. However, little is known about how cells handle potentially toxic protein aggregates. Here we demonstrate that cells are capable of clearing preformed alpha-synuclein aggregates via the lysosomal degradation pathway. Consequently, blocking this pathway causes the accumulation of the aggregates in non-neuronal cells, differentiated neuroblastoma cells, and primary cortical neurons. This aggregate clearance occurs in an aggregation stage-specific manner; oligomeric intermediates are susceptible to clearance, whereas mature fibrillar inclusion bodies are not. Neutralization of the acidic compartments leads to the accumulation of alpha-synuclein aggregates and exacerbates alpha-synuclein toxicity in postmitotic neuronal cells, suggesting that the accumulation of oligomeric intermediates may be an important event leading to alpha-synuclein-mediated cell death. These results suggest that enhancing lysosomal function may be a potential therapeutic strategy to halt or even prevent the pathogenesis of Parkinson's disease and other Lewy body diseases.
Figures

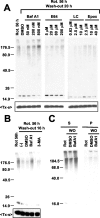
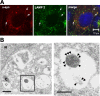
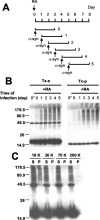
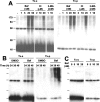



References
-
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y (1997) Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol 12: 25-31. - PubMed
-
- Bahr BA, Bendiske J (2002) The neuropathogenic contributions of lysosomal dysfunction. J Neurochem 83: 481-489. - PubMed
-
- Bendiske J, Caba E, Brown QB, Bahr BA (2002) Intracellular deposition, microtubule destabilization, and transport failure: an “early” pathogenic cascade leading to synaptic decline. J Neuropathol Exp Neurol 61: 640-650. - PubMed
-
- Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM (1999) Degradation of α-synuclein by proteasome. J Biol Chem 274: 33855-33858. - PubMed
-
- Biederbick A, Kern HF, Elsasser HP (1995) Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol 66: 3-14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
