L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10
- PMID: 14985440
- PMCID: PMC6730397
- DOI: 10.1523/JNEUROSCI.1670-03.2004
L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10
Abstract
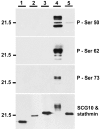
During development, EphB proteins serve as axon guidance molecules for retinal ganglion cell axon pathfinding toward the optic nerve head and in midbrain targets. To better understand the mechanisms by which EphB proteins influence retinal growth cone behavior, we investigated how axon responses to EphB were modulated by laminin and L1, two guidance molecules that retinal axons encounter during in vivo pathfinding. Unlike EphB stimulation in the presence of laminin, which triggers typical growth cone collapse, growth cones co-stimulated by L1 did not respond to EphB. Moreover, EphB exposure in the presence of both laminin and L1 resulted in a novel growth cone inhibition manifested as a pause in axon elongation with maintenance of normal growth cone morphology and filopodial activity. Pauses were not associated with loss of growth cone actin but were accompanied by a redistribution of the microtubule cytoskeleton with increased numbers of microtubules extending into filopodia and to the peripheral edge of the growth cone. This phenomenon was accompanied by reduced levels of the growth cone microtubule destabilizing protein SCG10. Antibody blockade of SCG10 function in growth cones resulted in both changes in microtubule distribution and pause responses mirroring those elicited by EphB in the presence of laminin and L1. These results demonstrate that retinal growth cone responsiveness to EphB is regulated by co-impinging signals from other axon guidance molecules. Furthermore, the results are consistent with EphB-mediated axon guidance mechanisms that involve the SCG10-mediated regulation of the growth cone microtubule cytoskeleton.
Figures



Similar articles
-
Retinal axon growth cones respond to EphB extracellular domains as inhibitory axon guidance cues.Development. 2001 Aug;128(15):3041-8. doi: 10.1242/dev.128.15.3041. Development. 2001. PMID: 11532925
-
Invariant Sema5A inhibition serves an ensheathing function during optic nerve development.Development. 2003 Feb;130(4):775-84. doi: 10.1242/dev.00299. Development. 2003. PMID: 12506007
-
The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones.J Neurosci. 2001 Dec 1;21(23):9194-203. doi: 10.1523/JNEUROSCI.21-23-09194.2001. J Neurosci. 2001. PMID: 11717353 Free PMC article.
-
Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth.J Neurobiol. 2004 Jan;58(1):60-9. doi: 10.1002/neu.10279. J Neurobiol. 2004. PMID: 14598370 Review.
-
Microtubules and growth cone function.J Neurobiol. 2004 Jan;58(1):70-83. doi: 10.1002/neu.10266. J Neurobiol. 2004. PMID: 14598371 Review.
Cited by
-
Disease-associated mutations in human TUBB3 disturb netrin repulsive signaling.PLoS One. 2019 Jun 21;14(6):e0218811. doi: 10.1371/journal.pone.0218811. eCollection 2019. PLoS One. 2019. PMID: 31226147 Free PMC article.
-
Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease.J Cell Biol. 2012 Oct 29;199(3):437-51. doi: 10.1083/jcb.201203109. J Cell Biol. 2012. PMID: 23109669 Free PMC article.
-
Secreted frizzled related proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms.J Neurosci. 2015 Mar 18;35(11):4729-40. doi: 10.1523/JNEUROSCI.3304-13.2015. J Neurosci. 2015. PMID: 25788689 Free PMC article.
-
EphB2 and EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis and branching remodeling.Blood. 2006 Nov 1;108(9):2914-22. doi: 10.1182/blood-2006-05-023341. Epub 2006 Jul 13. Blood. 2006. PMID: 16840724 Free PMC article.
-
ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells.Development. 2008 Jun;135(11):1981-90. doi: 10.1242/dev.010751. Epub 2008 Apr 23. Development. 2008. PMID: 18434421 Free PMC article.
References
-
- Antonsson B, Montessuit S, Di Paolo G, Lutjens R, Grenningloh G (1997) Expression, purification, and characterization of a highly soluble N-terminal-truncated form of the neuron-specific membrane-associated phosphoprotein SCG10. Protein Expr Purif 9: 295-300. - PubMed
-
- Antonsson B, Kassel DB, Di Paolo G, Lutjens R, Riederer BM, Grenningloh G (1998) Identification of in vitro phosphorylation sites in the growth cone protein SCG10. Effect Of phosphorylation site mutants on microtubule-destabilizing activity. J Biol Chem 273: 8439-8446. - PubMed
-
- Antonsson B, Kassel DB, Ruchti E, Grenningloh G (2001) Differences in phosphorylation of human and chicken stathmin by MAP kinase. J Cell Biochem 80: 346-352. - PubMed
-
- Bartsch U, Kirchhoff F, Schachner M (1989) Immunohistochemical localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. J Comp Neurol 284: 451-462. - PubMed
-
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M (2000) Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development 127: 1231-1241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
