Monitoring clathrin-mediated endocytosis during synaptic activity
- PMID: 14985443
- PMCID: PMC6730391
- DOI: 10.1523/JNEUROSCI.4080-03.2004
Monitoring clathrin-mediated endocytosis during synaptic activity
Abstract
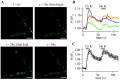
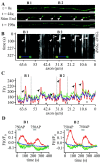
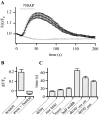
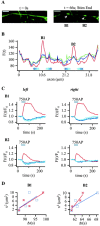
To visualize clathrin redistribution during endocytosis in hippocampal boutons, we used a fusion protein of clathrin light chain with enhanced green fluorescent protein. Both high potassium and electric field stimulation lead after a stimulus-dependent delay to a transient increase of fluorescence in synapses, but a slight and transient decrease in adjacent axonal segments. We conclude that the rise and fall of the signal in boutons, with decay kinetics remarkably similar to previous estimates of the endocytic time course, reflects coat assembly and disassembly. Thus, we could selectively measure clathrin-mediated endocytosis and separate its kinetics from other modes of membrane retrieval in CNS synapses. A long-lasting delay preceding the fluorescent transients shows that endocytosis during the first few seconds of continuing stimulation cannot be mediated by newly formed clathrin-coated pits. Therefore, a fast mode of endocytosis is either clathrin-independent or involves preassembled (easily retrievable) clathrin lattices at sites of endocytosis.
Figures



References
-
- Aravanis AM, Pyle JL, Tsien RW (2003) Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423: 643-647. - PubMed
-
- Ashery U, Betz A, Xu T, Brose N, Rettig J (1999) An efficient method for infection of adrenal chromaffin cells using the Semliki-Forest virus gene expression system. Eur J Cell Biol 78: 525-532. - PubMed
-
- Blanpied TA, Scott DB, Ehlers MD (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36: 435-449. - PubMed
-
- Brodin L, Low P, Shupliakov O (2000) Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol 10: 312-320. - PubMed
-
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE (2001) Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol 17: 517-568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
