The number of glutamate receptors opened by synaptic stimulation in single hippocampal spines
- PMID: 14985448
- PMCID: PMC6730404
- DOI: 10.1523/JNEUROSCI.5066-03.2004
The number of glutamate receptors opened by synaptic stimulation in single hippocampal spines
Abstract
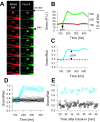
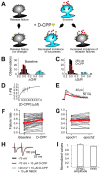
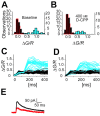
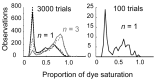
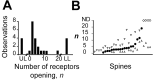
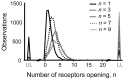
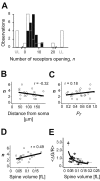
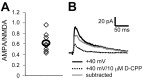
The number of receptors opening after glutamate release is critical for understanding the sources of noise and the dynamic range of synaptic transmission. We imaged [Ca2+] transients mediated by synaptically activated NMDA receptors (NMDA-Rs) in individual spines in rat brain slices. We show that Ca2+ influx through single NMDA-Rs can be reliably detected, allowing us to estimate the number of receptors opening after synaptic transmission. This number is small: at the peak of the synaptic response, less than one NMDA-R is open, on average. Therefore, stochastic interactions between transmitter and receptor contribute substantially to synaptic noise, and glutamate occupies a small fraction of receptors. The number of receptors opening did not scale with spine volume, and smaller spines experience larger [Ca2+] transients during synaptic transmission. Our measurements further demonstrate that optical recordings can be used to study single receptors in intact systems.
Figures
References
-
- Bading H, Ginty DD, Greenberg ME (1993) Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260: 181-186. - PubMed
-
- Bekkers JM, Stevens CF (1989) NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature 341: 230-233. - PubMed
-
- Casado M, Isope P, Ascher P (2002) Involvement of presynaptic N-methyl-d-aspartate receptors in cerebellar long-term depression. Neuron 33: 123-130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
