Kinesin's second step
- PMID: 14985504
- PMCID: PMC373481
- DOI: 10.1073/pnas.0307691101
Kinesin's second step
Abstract
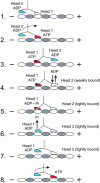
We have identified dimeric kinesin mutants that become stalled on the microtubule after one ATP turnover, unable to bind and hydrolyze ATP at their second site. We have used these mutants to determine the regulatory signal that allows ATP to bind to the forward head, such that processive movement can continue. The results show that phosphate release occurs from the rearward head before detachment, and detachment triggers active-site accessibility for ATP binding at the forward head. This mechanism, in which the rearward head controls the behavior of the forward head, may be conserved among processive motors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

