Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid
- PMID: 14985508
- PMCID: PMC373470
- DOI: 10.1073/pnas.0400282101
Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid
Abstract
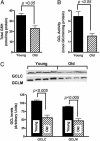
Glutathione (GSH) significantly declines in the aging rat liver. Because GSH levels are partly a reflection of its synthetic capacity, we measured the levels and activity of gamma-glutamylcysteine ligase (GCL), the rate-controlling enzyme in GSH synthesis. With age, both the catalytic (GCLC) and modulatory (GCLM) subunits of GCL decreased by 47% and 52%, respectively (P < 0.005). Concomitant with lower subunit levels, GCL activity also declined by 53% (P < 0.05). Because nuclear factor erythroid2-related factor 2 (Nrf2) governs basal and inducible GCLC and GCLM expression by means of the antioxidant response element (ARE), we hypothesized that aging results in dysregulation of Nrf2-mediated GCL expression. We observed an approximately 50% age-related loss in total (P < 0.001) and nuclear (P < 0.0001) Nrf2 levels, which suggests attenuation in Nrf2-dependent gene transcription. By using gel-shift and supershift assays, a marked reduction in Nrf2/ARE binding in old vs. young rats was noted. To determine whether the constitutive loss of Nrf2 transcriptional activity also affects the inducible nature of Nrf2 nuclear translocation, old rats were treated with (R)-alpha-lipoic acid (LA; 40 mg/kg i.p. up to 48 h), a disulfide compound shown to induce Nrf2 activation in vitro and improve GSH levels in vivo. LA administration increased nuclear Nrf2 levels in old rats after 12 h. LA also induced Nrf2 binding to the ARE, and, consequently, higher GCLC levels and GCL activity were observed 24 h after LA injection. Thus, the age-related loss in GSH synthesis may be caused by dysregulation of ARE-mediated gene expression, but chemoprotective agents, like LA, can attenuate this loss.
Figures



References
-
- Harman, D. (1992) Mutat. Res. 275, 257-266. - PubMed
-
- Beckman, K. B. & Ames, B. N. (1998) Physiol. Rev. 78, 547-581. - PubMed
-
- Liu, R. & Choi, J. (2000) Free Radical Biol. Med. 28, 566-574. - PubMed
-
- Uejima, Y., Fukuchi, Y., Teramoto, S., Tabata, R. & Orimo, H. (1993) Mech. Ageing Dev. 67, 129-139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

