Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior
- PMID: 14988500
- PMCID: PMC373512
- DOI: 10.1073/pnas.0308753101
Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior
Abstract
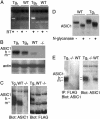
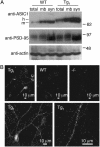
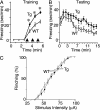
The acid-sensing ion channel 1a (ASIC1a) is abundantly expressed in the amygdala complex and other brain regions associated with fear. Studies of mice with a disrupted ASIC1 gene suggested that ASIC1a may contribute to learned fear. To test this hypothesis, we generated mice overexpressing human ASIC1a by using the pan-neuronal synapsin 1 promoter. Transgenic ASIC1a interacted with endogenous mouse ASIC1a and was distributed to the synaptosomal fraction of brain. Transgenic expression of ASIC1a also doubled neuronal acid-evoked cation currents. The amygdala showed prominent expression, and overexpressing ASIC1a enhanced fear conditioning, an animal model of acquired anxiety. These data raise the possibility that ASIC1a and H(+)-gated currents may contribute to the development of abnormal fear and to anxiety disorders in humans.
Figures

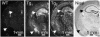
References
-
- Lecrubier, Y. (2001) J. Clin. Psychiatry 62, 4-9. - PubMed
-
- Gorman, J. M., Kent, J. M., Sullivan, G. M. & Coplan, J. D. (2000) Am. J. Psychiatry 157, 493-505. - PubMed
-
- Kim, J. J., Rison, R. A. & Fanselow, M. S. (1993) Behav. Neurosci. 107, 1093-1098. - PubMed
-
- LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. - PubMed
-
- Maren, S. (2001) Annu. Rev. Neurosci. 24, 897-931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

